 |
 |
 |
| |
Pegasys+Lamivudine for HBeAg-negative, 2 Year Follow-up
|
| |
| |
Reported by Jules Levin
AASLD, Oct 27-31, 2006, Boston, MA
Suppression of HBV DNA in patients with HBeAg-negative CHB treated with peginterferon alfa-2a (40KD) ± lamivudine: 2-year follow-up results
Patrick Marcellin,1 Ferruccio Bonino,2 George KK Lau,3 Patrizia Farci,4 Cihan Yurdaydin,5 Teerha Piratvisuth,6 Kangxian Luo,7 Selim Gurel,8 Stephanos Hadziyannis,9 Yuming Wang,10 Matei Popescu11
1Service d'Hepatologie, H˘pital Beaujon, University of Paris, Clichy, France; 2Fondazione Istituto di Ricovero e Cura a Carattere Scientifico, Ospedale Maggiore di Milano Policlinico, Milan, Italy; 3Department of Medicine, Queen Mary Hospital, Hong Kong, China; 4Dipartimento di Scienze Mediche, UniversitÓ di Cagliari, Monserrato, Italy;
5Department of Gastroenterology, University of Ankara, Turkey; 6Department of Internal Medicine, Songklanagarind Hospital, Prince of Songkla University, Hat Yai, Thailand; 7Department of Infectious Diseases, Nanfang Hospital, Guangzhou, China; 8Department of Gastroenterology, Uludag University, Turkey; 9Department of Medicine and Hepatology, Henry Dunant Hospital, Athens, Greece;
10Infectious Diseasse Department, Xinan Hospital, Chongqin, China; 11Roche, Basel, Switzerland
Background
HBeAg-negative chronic hepatitis B (CHB) is a severe and progressive disease associated with a poor prognosis and low rates of response to therapy1
Treatment of HBeAg-negative CHB is difficult as patients are prone to high rates of treatment relapse, particularly following nucleos(t)ide analog therapy2,3
The durability of response to conventional interferon alfa therapy in HBeAg-negative disease is reported to be low, at only 20-25%4
The majority of patients who experience treatment relapse following conventional interferon alfa therapy do so within the first 12 months of follow-up5-7
A large, randomised study of patients with HBeAg-negative CHB showed that patients receiving peginterferon alfa-2a (40KD) (PEGASYS) alone or combined with lamivudine, achieved significantly higher response rates 24 weeks post-treatment than with lamivudine alone8
OBJECTIVE
To evaluate the durability of responses to PEGASYS, both as monotherapy
and in combination with lamivudine, in patients with HBeAg-negative CHB
over a period of up to 2 years post-treatment.
SUMMARY
More than one third of the patients treated with PEGASYS alone or in combination with lamivudine achieved a biochemical response sustained for
up to 2 years post-treatment
Over one quarter of patients maintained post-treatment HBV DNA levels below 104 cp/mL (10,000), a viral load that has been identified as being associated with a marked reduction in the risk of progressive liver disease and hepatocellular carcinoma9
Among PEGASYS-treated patients who had normal ALT 6 months post-treatment 76% maintained this response 1 year post-treatment HBV DNA levels ≦20,000 cp/mL, the virologic endpoint in the initial study, were sustained by 73% of patients 2 years post-treatment
Virologic response rates remained stable from 9 months to 2 years post-treatment; patients who relapsed, did so in the early months of follow-up
Persistent HBsAg loss and/or seroconversion during the post-treatment period was achieved by approximately 5% of patients
Addition of lamivudine to PEGASYS did not provide an advantage in terms of sustained response
CONCLUSION
A finite (48-week) course of PEGASYS (± lamivudine) induced biochemical and
HBV DNA (≦10,000 cp/mL) responses in around one third of the patients that
were sustained for up to 2 years post-treatment. The ability of PEGASYS to induce biochemical and virologic responses that are sustained 2 years post-treatment without the need for long-term therapy, and the associated risk of drug resistance, support its use as a first-line treatment of HBeAg-negative CHB
Initial study
In the initial study,8 conducted at 54 sites in 13 countries, patients with
HBeAg-negative CHB were randomized to one of the following treatment
groups for 48 weeks:
-- PEGASYS 180 _g once weekly plus oral placebo daily (n=177)
-- PEGASYS 180 _g once weekly plus lamivudine 100 mg daily (n=179)
-- Lamivudine 100 mg daily (n=181)
Co-primary efficacy outcomes assessed 24 weeks post-treatment were:
-- Normalization of ALT to <1 x the upper limit of normal (ULN)
-- HBV DNA suppression <20,000 cp/mL
All patients were positive for HBsAg and negative for anti-HBs antibody at
baseline
Long-term (LT) study
All centres involved in the initial study were offered participation in a roll-over 5-year long-term observational study designed to assess the durability of response
Out of 54 centres, 42 accepted participation in the LT study; 315 out of 537 patients (59%) originally involved in the three treatment arms were included in
the roll-over study
The number of lamivudine-treated patients (85/181, 47%) participating in the LT study was significantly (P<0.01) less than those treated with PEGASYS monotherapy (116/177, 66%) or PEGASYS plus lamivudine (114/179, 64%). Therefore, for this analysis, we evaluated the durability of response to PEGASYS ± lamivudine only
Endpoints assessed 2 years post-treatment were:
-- ALT normalization (≦30 IU/L complete response or >30-≦50 IU/L partial)
-- HBV DNA suppression <100,00 cp/mL, <10,000 cp/mL and <400 cp/mL
-- ALT and HBV DNA were measured at weeks 48, 52, 56, 60, 64, 72, 84 100 and 152. HBV DNA levels were measured using the Roche COBAS AMPLICOR HBV DNA Monitor Test (Roche Diagnostics)
RESULTS
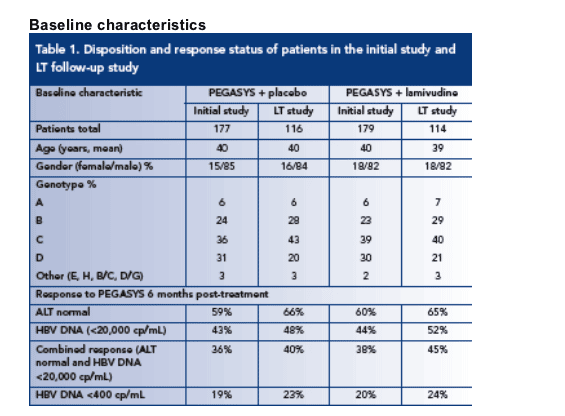
The baseline demographics of PEGASYS-treated patients were similar in the initial and LT studies (Table 1)
The genotypic distribution of patients participating in the initial and LT studies were comparable; the majority (60-70%) of patients being infected with HBV genotypes B or C
The proportion of responders participating in the LT study was not significantly different from the proportion of responders in the initial study (Table 1)
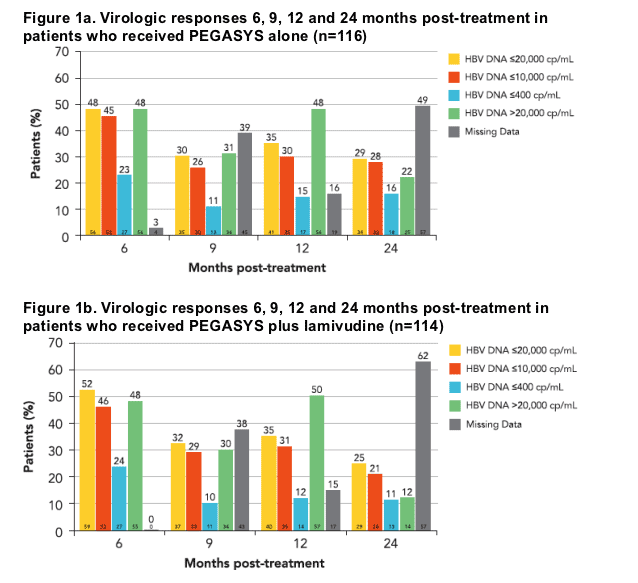
Virologic and biochemical response 6, 9, 12 and 24 months post-treatment
The number of patients achieving HBV target levels (<10,000 and <400 cp/mL) was similar for both treatment arms at 1 and 2 years posttreatment (Figures 1a and 1b)
At 1 year post-treatment, 30% and 31% achieved HBV DNA levels of <10,000
cp/mL and 15% and 12% had <400 cp/mL for those patients receiving PEGASYS alone (Figure 1a) and the combination with lamivudine (Figure 1b), respectively
At 2 years post-treatment, the proportions observed were 28% and 21% for
<10,000 cp/mL and 16% and 11% for <400 cp/mL, for PEGASYS alone and
the combination with lamivudine, respectively
Overall, the virologic response remained stable from 9 months through to
2 years post-treatment
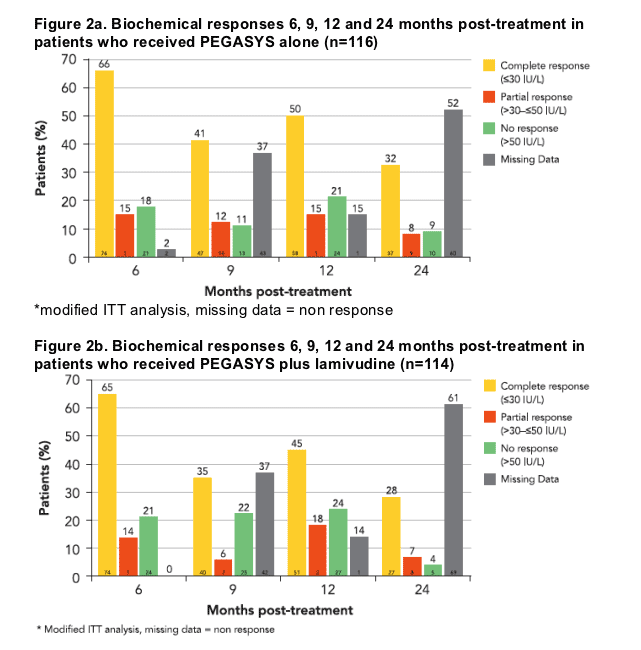
1 year post-treatment, 50% of patients treated with PEGASYS alone had normal ALT levels (ALT ≦30 IU/L); 15% of patients had mildly elevated ALT (>30 but ≦50 IU/L). At 2 years post-treatment 40% of patients had normal or mildly elevated ALT levels (Figure 2a)
For those treated with PEGASYS plus lamivudine, 45% of patients had normal ALT levels; 18% of patients had mildly elevated ALT 1 year post-treatment. At 2 years post-treatment, 35% of patients had normal or mildly elevated ALT levels (Figure 2b)
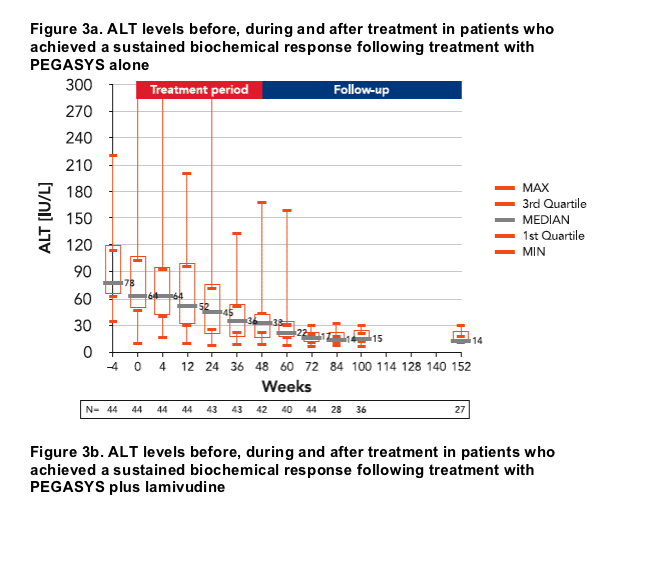
HBsAg loss and seroconversion
Of the patients treated with PEGASYS alone, 11/116 lost HBsAg during the
Study:
-- 6 patients developed anti-HBs antibodies which were maintained over 2
years of follow-up
-- 1 patient lost HBsAg 2 years post-treatment
-- 4 patients with HBsAg loss, who did not develop anti-HBs, reverted to
HBsAg-positive status
In the PEGASYS + lamivudine treatment arm, 14/114 patients lost HBsAg
during the study:
-- 6 patients developed anti-HBs antibodies during the study
-- 6 patients maintained HBsAg loss over 2 years of follow-up with 2
developing anti-HBs antibodies
|
| |
|
 |
 |
|
|