 |
 |
 |
| |
Pegasys + Adefovir Reduces HBsAg in HDV-Coinfected
|
| |
| |
Reported by Jules Levin
AASLD, Oct 27-31, 2006, Boston, MA
Pegylated interferon alfa-2a plus adefovir combination therapy is superior to pegylated interferon alfa-2a alone or adefovir monotherapy in reducing HBsAg levels in HDV-coinfected patients with low HBV viremia
H. Wedemeyer,1 C. Yurdaydin,2 K. Zachou,1,3 A. Erhardt,4 U. Drebber,5 Y. Cakaloglu,6 H. Degertekin,7 S. Gurel,8 S. Zeuzem,9 T. Bock,10 G. Dalekos,3 M. P. Manns1
1Gastroenterology, Medizinische Hochschule Hannover, Hannover , Germany; 2Ankara University, Ankara, Turkey; 6Istanbul University, Istanbul, Turkey; 7Dicle University School of Medicine, Diyarbakir, Turkey; 8Bursa University, Bursa, Turkey;
9University of Saarland, Homburg, Germany; 10University of Tübingen, Tübingen, Germany; 5University of Cologne, Cologne, Germany; 4University of Duesseldorf, Duesseldorf, Germany; 3University of Larissa, Larissa, Greece
BACKGROUND
Serum HBsAg levels are considered as a surrogate marker for intrahepatic HBV-cccDNA levels and have been shown to be\ reduced by adefovir therapy in HBV-monoinfected patients
The hepatitis D virus (HDV) uses the HBsAg for virion formation. Thus, the best treatment option for HDV infection would be clearance of HBsAg
So far, there are no data available on HBsAg levels during pegylated interferon alfa therapy alone or in combination with nucleos(t)ides in HBV infection
SUMMARY AND CONCLUSIONS
This is the fi rst trial demonstrating that a combination of pegylated interferon with a nucleotide is superior to either monotherapy in reducing HBsAg levels in HBV-infected patients
HBsAg clearance can be achieved by combination therapy in some HDV-coinfected patients after only 48 weeks of therapy
Future trials are required to investigate whether longer treatment regimes can increase HBsAg seroconversion rates in HDV-coinfected as well as in HBV-monoinfected patients
METHODS
Patients with HBV-HDV co-infection (n=90) enrolled in the Hep-Net/International Delta Hepatitis Intervention Trial (HID-IT) (Figure 1), carried out at centres in Germany, Turkey and Greece, were treated for 48 weeks with:
-- 180 _g peginterferon alfa-2a (PEGASYS) qw plus 10 mg adefovir
dipivoxil qd (group A, n=31)
-- 180 _g PEGASYS qw plus placebo (group B, n=29)
-- 10 mg adefovir dipivoxil qd alone (group C, n=30)
HBsAg was measured at baseline and at the end of treatment (week 48). HBsAg levels were determined using the Abbott Architect System. Results are expressed in International Units HDV-RNA levels were determined by in house Real-Time PCR
At week 48, HDV-RNA was negative in 19%, 30% and 12% of patients in the HID-IT trial, respectively
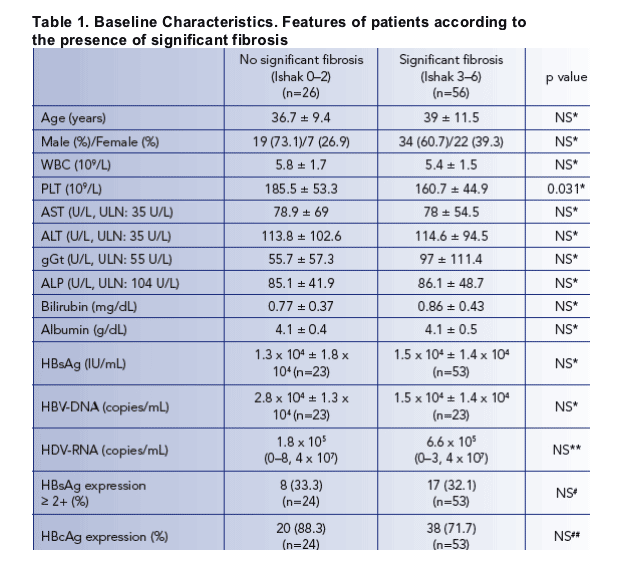
BASELINE CHARACTERISTICS
At baseline 14 patients tested positive for HBeAg, 48% of patients had HBV-DNA levels below 100 IU/mL, 37% had a HBV viremia between 101 and 10,000 IU/mL and 15% showed HBV-DNA levels above 10,000 IU/mL
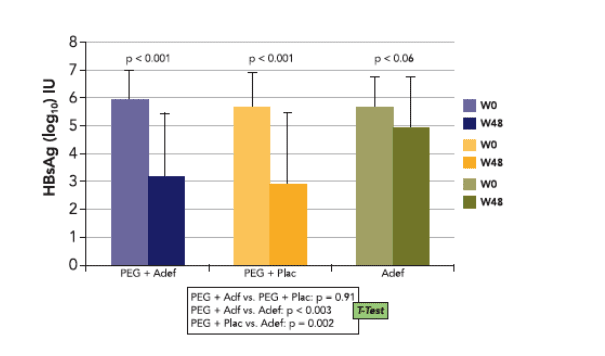
Mean baseline HBsAg levels were 3.9±0.6, 4.0±0.6 and 4.0±0.5 log10 IU/mL in groups A, B and C, respectively, ranging from 184 to 79591 IU/mL
HBsAg levels did not correlate with HBV viremia but were positively correlated with HDV-RNA levels (r=0.324, p=0.002)
RESULTS
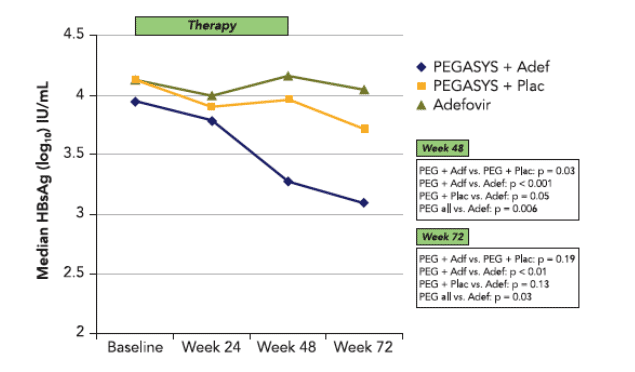
While patients receiving PEGASYS alone or adefovir monotherapy had similar mean HBsAg levels at week 0 and week 48, the PEGASYS/ adefovir combination group showed a 1.0 log10 IU/mL decline of HBsAg levels by week 48 (p<0.001).
(This must be a misprint: since peg/ADV performed better they must mean 40% in group B):
40% of patients in group A but only 5% of patients in group B displayed at least a 10-fold reduction of HBsAg in serum compared with none of the patients in group C (p<0.001).
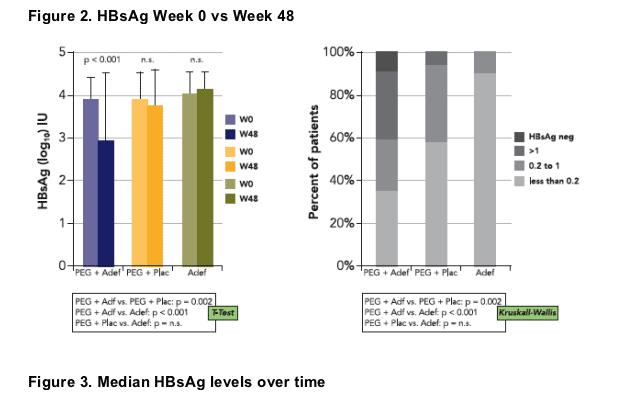
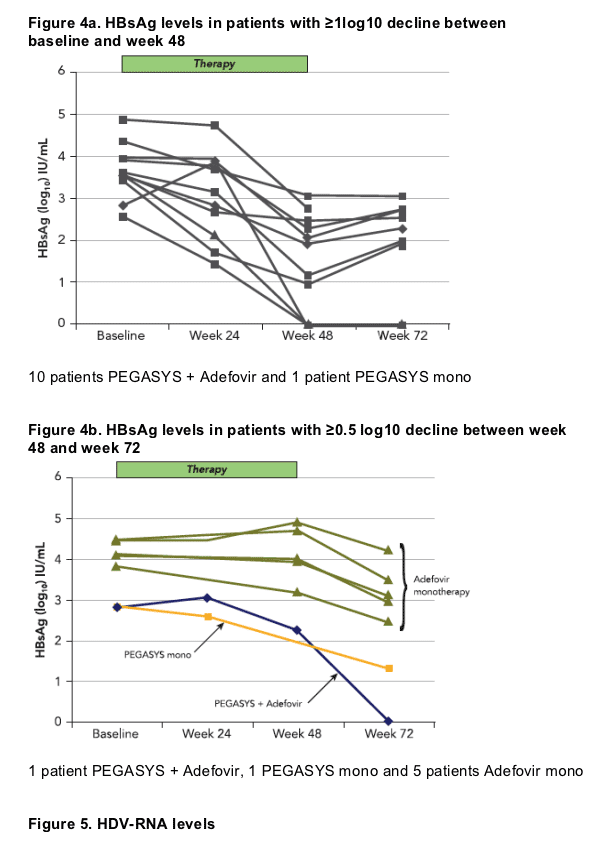
HBsAg was lost by week 48 in 2 patients, both patients were treated with a combination of PEGASYS and adefovir and had also developed anti-HBs antibodies with titers of 58 and 413 IU/L, respectively 1 additional patient lost HBsAg during follow-up (PEGASYS + adefovir group)
A significant HBsAg decline was not seen before week 24 in most responding patients (Figure 4). The response was maintained in most patients after the end of therapy with a <1 log10 increase until week 72 in 5 out of 10 patients
Median HBsAg levels remained >1000 IU/mL in the majority of patients after 48 weeks even in patients receiving combination therapy, suggesting that longer therapies will required in most patients to achieve HBsAg loss. Patients who could potentially benefi t from longer treatment may be identifi ed by measuring HBsAg kinetics
The complete data set on HDV-RNA and ALT levels will be presented in the oral presentation by Prof. Yurdaydin at AASLD 2006 Abstract No. 111
This research was partly funded by Roche, Basel, Switzerland and Gilead
Sciences, Germany
|
| |
|
 |
 |
|
|