 |
 |
 |
| |
Next Generation HIV Peptide Fusion Inhibitor Candidates Achieve Potent, Durable Suppression of Virus replication in vitro and Improved Pharmacokinetic Properties
|
| |
| |
Reported by Jules Levin
13th CROI (Retrovirus Conference), Denver, Feb 6, 2006
Mary Kay Delmedico, Brian L Bray, Nick Cammack, Donna K Davison, John J Dwyer, Lloyd W Frick, Nicolai A Tvermoes, Stephen A wring, Huyi Zhang, Michael L Greenberg, Trimeris, Roche
Enfuvirtide (Fuzeon, T-20), the first approved entry inhibitor for HIV and an important therapeutic for treatment-experienced patients, is administered as a subcutaneous injection twice a day. The goal for the next-generation fusion inhibitor candidate is to maintain or improve upon the efficacy demonstrated by ENF while decreasing injection frequency. Trimeris researchers have identified 2 candidates that demonstrate substantial improvements in potency, durability, and pharmacokinetics. These peptide candidates are being evaluated with sustained-release formulations targeting once/week administration.
Mary Kay Delmedico from Trimeris presented these data and information today in an oral presentation at the 10am session on "Antiretroviral Therapy I: New Agents and New Insights".
Fuazeon is
The first generation entry inhibitor
First peptide for treatment of HIV disease
Low potential for systemic side effects or drug-drug interactions
Fully active against multi-drug resistant HIV
Active against R5, X4, and dual-mixed viruses
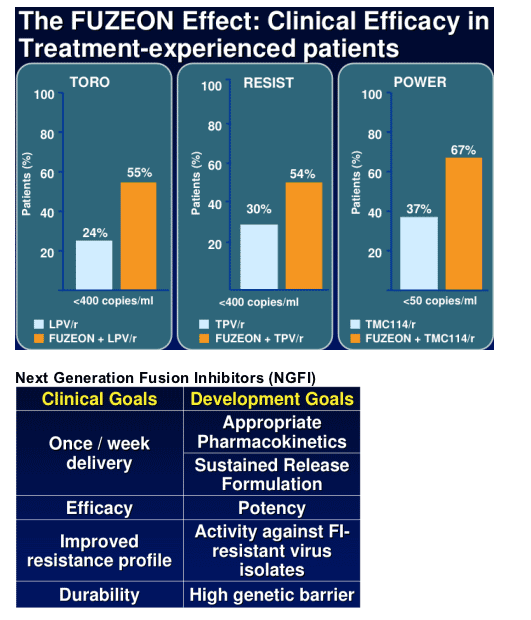
Treatment-experienced patients are 2-fold more likely to achieve "undetectable'
when ENF is combined with other active agents
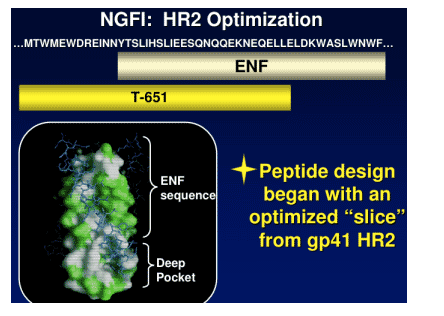
Research for the 2 new candidates started with T-651, which was subjected to redesign to find drug candidates that meet the goals mentioned just above. This process took 1 to 1.5 years and 20 researchers at Trimeris.
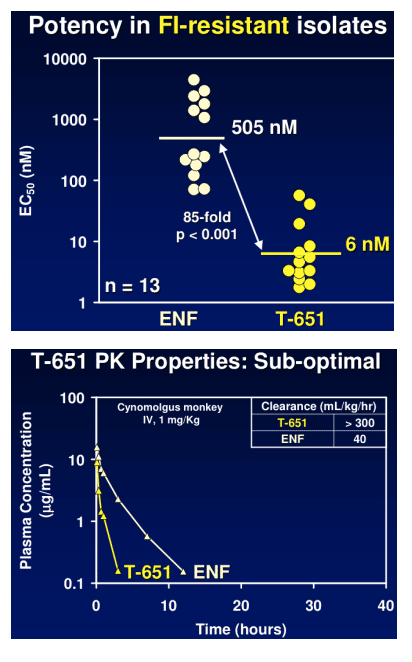
Candidate potency has been evaluated in a cMAGI assay against a range of laboratory and clinical isolates, having varying degrees of sensitivity to ENF. Durability of the peptides has been evaluated in in vitro passaging experiments. Intravenous and subcutaneous pharmacokinetic parameters have been obtained in cynomolgus monkeys.
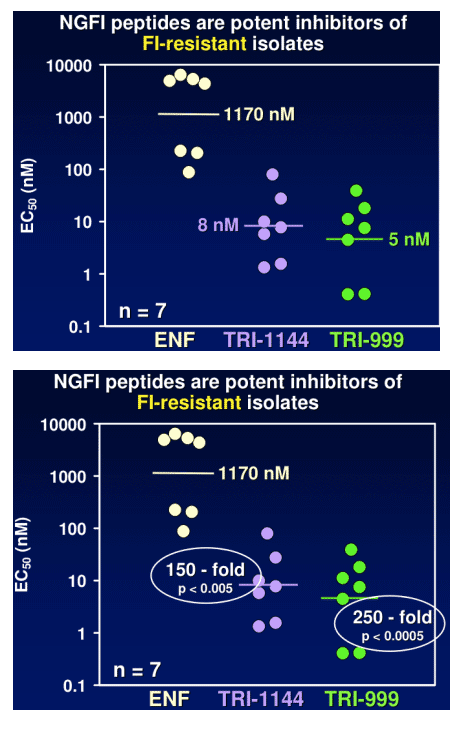
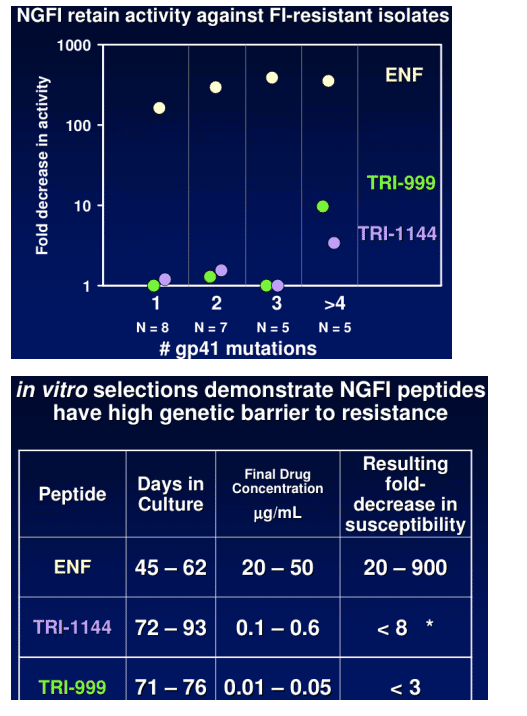
TR-290999 and TR-291144 are peptides derived from a gp41 HR2 region partially overlapping the ENF sequence. Both peptides have been modified using independent strategies to optimize potency, durability, and pharmacokinetic properties. TR-291144 displays potent antiviral activity against a panel of 12 clinical isolates, with a 7-nM geometric mean IC50 equivalent to the performance of ENF. TR-290999 displays a 7-fold improvement over Fuzeon against this panel, with a geometric mean IC50 of 1 nM. Both compounds have potent activity against an isolate panel resistant to Fuzeon, T-1249, and other peptide fusion inhibitors. Passaging experiments demonstrate superior in vitro durability of these compounds compared to other peptide fusion inhibitors. Cynomlogus monkey intravenous clearance values for TR-290999 and TR-291144 are 4 and 9 mL/Kg/hr, respectively, equal to 10- and 4-fold improvements over ENF. Subcutaneous bioavailability values for TR-290999 and TR-291144 are 100% and 87%, respectively.
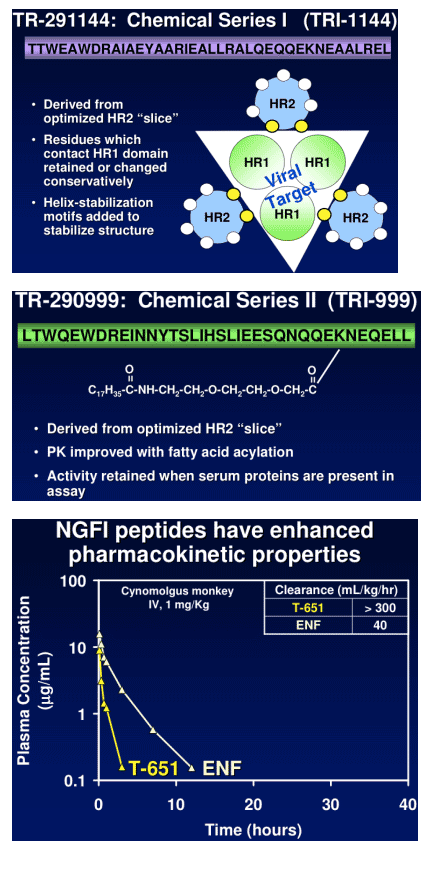
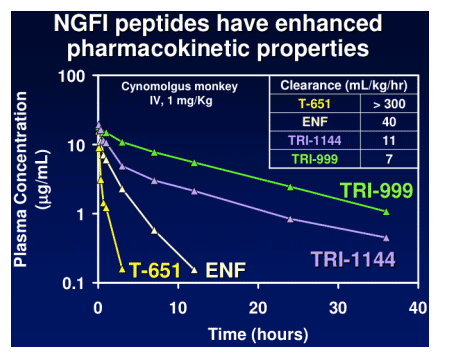
NGFI Pharmacokinetics
NGFI peptides have high sub-cutaneous bioavailability: cynomolgus monkeys = 85-90%.
NGFI peptides have PK properties that are amenable to once/week dosing with appropriate sustained release formulations.
Current Work
Developing sustained-release formulation for once/week delivery
Progressing towards clinical studies in patients.
|
|
| |
| |
|
 |
 |
|
|