 |
 |
 |
| |
Effect of pioglitazone on HIV-1 lipoatrophy : a randomised, double-bind, placebo-controlled trial (ANRS 113)
|
| |
| |
Reported by Jules Levin
Three studies of using glitazones and anti-diabetic drugs (pioglitazone, rosiglitazone, metformin) were presented in an oral session at the 13th CROI in Denver, Feb 5-8, 2006), but the study using pioglitazone showed benefit of improved lipoatrophy, while the other studies showed mixed results. Here is the first study using pioglitazone, & the other studies will follow. Another report with commentary for NATAP by David Wohl, MD is in preperation & will follow soon.
Study authors: Laurence Slama, Emilie Lanoy, Marc-Antoine Valentin, Jean Philippe Bastard, Aziza Chermak, Amal Boutekadjirt, Demiana William-Faltaos, Jacqueline Capeau, Dominique Costagliola, Willy Rozenbaum; ANRS, (Agence nationale de recherches sur le sida et les hepatitis virales)
Study aim:
To assess the efficacy, safety of pioglitazone 30 mg qd in lipoatrophic
HIV-1+ adults receiving antiretroviral therapy, and to measure the effects of this treatment on glucose and lipid metabolism, over 48 weeks.
Author Conclusion:
- Pioglitazone 30 mg qd for 48 weeks improved limb fat atrophy in antiretroviral-treated HIV-1 infected patients.
- This effect was not observed in patients exposed to stavudine
- Pioglitazone did not modify lipid profile except a favourable increase in HDL cholesterol.
- Pioglitazone was well tolerated without any adverse effects related to study drug.
- Although no improvement was perceived by patients, additive effect of a switch of thymidine analogues, longer follow-up as well as higher dose deserves further investigation.
In discussion with Dr Rozen baum at CROI I think he said he will be studying a higher dose of pioglitazone, 45 mg once daily.
Effect of Pioglitazone on Lipoatrophy
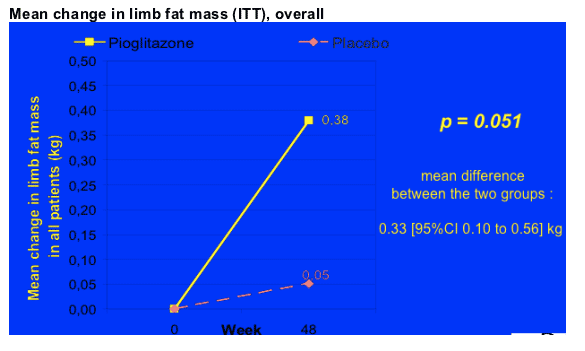
Mean Change in limb fat mass (ITT) (see pics of graphs below)
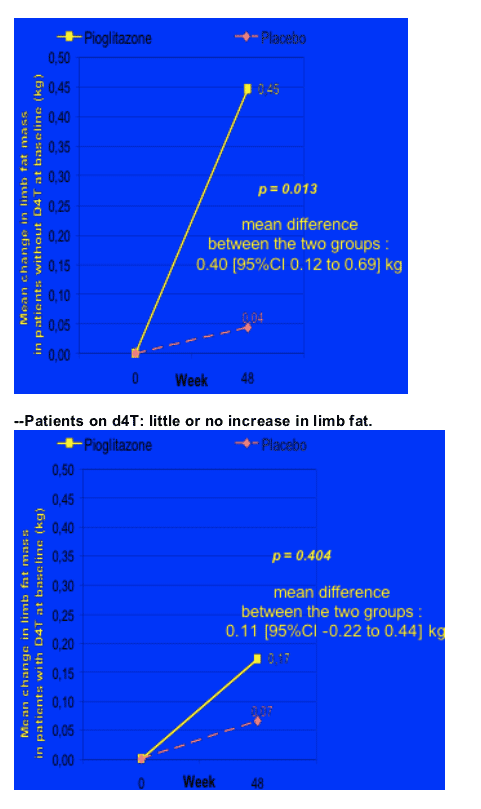
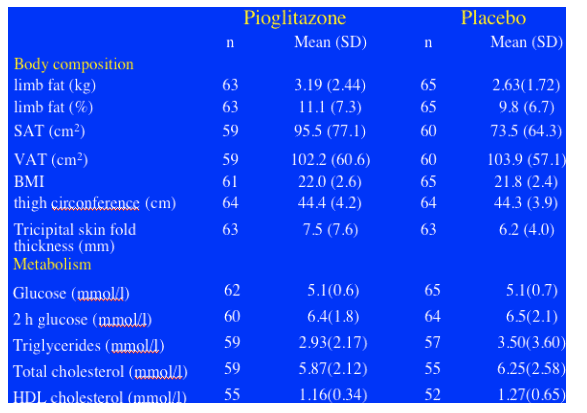
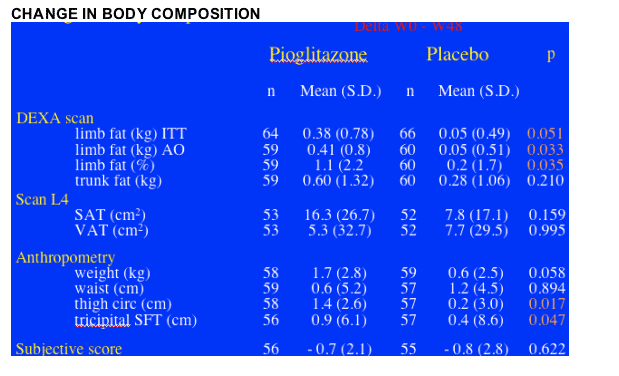
Patients not on d4T (n=94) who received pioglitazone achieved an increase in limb fat of 450g after 48 weeks of pioglitazone therapy (p=0.013) (ITT analysis). Patients on d4T at baseline (n=36) & pioglitazone saw little or no improvement (p=0.404). At baseline, mean limb fat was 3.19 kg for patients who received pioglitazone in the study and patients who received placebo had a mean 2.63 kg of limb fat. These were skinny patients with lipoatrophy, as the average limb fat for individuals is about 8 kg. In general you might expect a French population to be skinnier or weight= less than Americans. Rozenbaum said this was confirmed in the As-Observed analysis: limb fat increased by 1.1% in the pioglitazone group vs 0.2% in the placebo group (p=0.035), if this is true, a rather small but real improvement. There was an increase in thigh circumfrance and tricipital skin-fold thickness measure by anthropometry: thigh circumfrance increase 1.4 cm for patients on pioglitazone vs 0.2 cm for patients on placebo (p=0.017); tricipital SFT increased 0.9 cm for patients on pioglitazone vs 0.4 cm for patients on placebo (p=0.047). There was no difference in belly fat (VAT) change by DEXA between the pioglitazone & placebo groups, and there was no difference between the 2 groups in weight change or in waist, see table below. However, pioglitazone had no benefit when evaluated by patient subjective score, see table below.
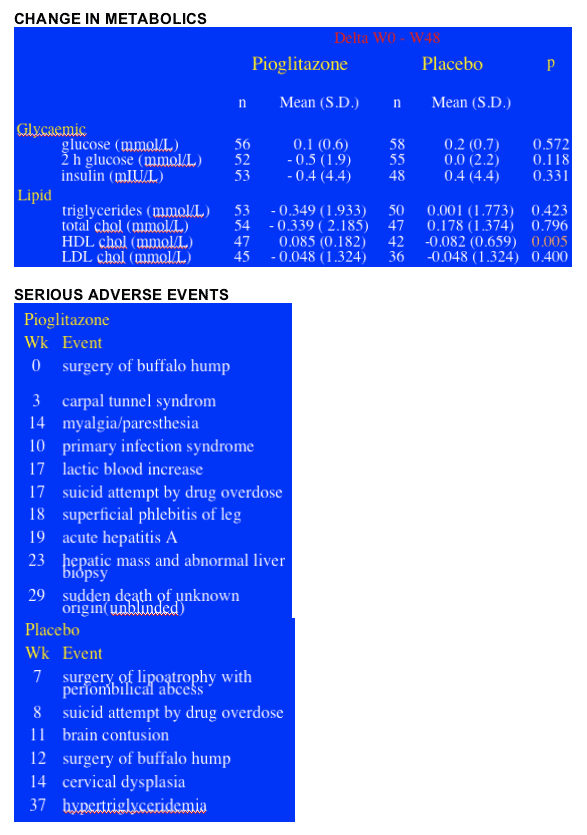
METABOLIC CHANGES: There were no significant differences in the mean changes between pioglitazone & placebo regarding glycemia and lipids, except for HDL “good” cholesterol, which increased in the pioglitazone group (+0.085 mmol/L vs -0.082; p = 0.005) compared with the placebo group. The percentage of patients with metabolic abnormalities did not change significantly, Rozenbaum said.
The mean CD4 count decreased by 52 in the pioglitazone group and by 12 in the placebo group, difference was not significant (p=0.172). There was a small difference, although not significant, in the rate of viral failure between the two groups at week 48: 6 (10%) in the pioglitazone group vs 1 (1.7%) in the placebo group (p=0.114).
SERIOUS ADVERSE EVENTS: There were 10 in the pioglitazone group & 6 in the placebo group, 2 in PIO group were to the same patient, but Rozenbaum said none of the SAEs were due to study drug, see table below.
After 48 weeks of pioglitazone, overall the patients receiving therapy had an increase of 380g of fat compared to 5 kg in the placebo group, which translates into a difference of 330 kg of fat increase between the pioglitazone group and the placebo group, p=0.051 (95% CI 0.10 to 0.56). You can see below the table showing the baseline levels of limb fat, which was a mean of 3.19 kg in the patients receiving pioglitazone and 2.63 kg in patients on placebo. BUT, for patients exposed to d4T at baseline the difference was more stark. For patients on d4T there was no improvement in limb fat & for patients NOT on d4T there was a difference, as you can see in the second picture below. For patients NOT on d4T the mean limb fat improvement was s statistically significant 450g (mean difference between the 2 groups: PIO vs plac. 0.40 [95% CI 0.12 to 0.69] kg), but for patients on d4T the change was +11g but this was not significant (mean difference between the 2 groups: 0.11 [95% CI -0.22 to 0.44] kg).
Mean change in limb fat mass with and without D4T at baseline
--Patients not on d4T: increase in limb fat of 450 g.
Study End points:
Primary : change in limb fat between week 0 and 48 (DEXA)
Secondary :- change in limb fat and trunk fat mass in percentage (DEXA)
- change in SAT and VAT (CT Scan)
- change in anthropometric variable
- change in glucose and lipid metabolism
- safety
- changes in subjective discomfort
Study design:
- Double-blind, placebo-controlled, multicentre, 48 weeks study
- Stratified by sex
- 80 % power to detect a 0,5 standard deviation of the change in DEXA-assessed limb fat mass between groups
- 0.325 kg (n = 65/arm)
- Primary end point analysed by intention to treat and LOCF
- Additional analyses for all variables performed on available data (« as observed »)
- Subgroup analysis according to stavudine therapy at baseline planned in the protocol
Study eligibility:
- HIV lipoatrophy self-reported by the patient and confirmed by the investigator
- HIV-1 RNA < 400 cop/ml for last 6 months
- Stable ART for the previous 6 months
- CD4 cells count > 200/mm3
- ALT < 2.5 x ULN
amylase, lipase, creatinine < 2 x ULN
haemoglobin > 9 g/dl, platelet count > 50000/mm3
- No active infection, cardiac failure, pregnancy, diabetic or steroid therapy, lipid lowering therapy
Patient Disposition
137 patients were interviewed for study. 133 were assessed for eligibility. And 130 randomized: 4 declined the study; 3 not included: 1 CD4 cell count <200; 1 unstable ARV; 1 portal hypertension.
64 to the pioglitazone arm:
5 were lost to analysis: 1stopped the trial; 1 died; 3 stopped pioglitazone. 59 patients had validated DEXA at week 0 and week 48.
66 were randomized to placebo:
7 were lost to analysis: 5 stopped trial; 2 stopped placebo. 60 patients had validated DEXA at week 0 and week 48.
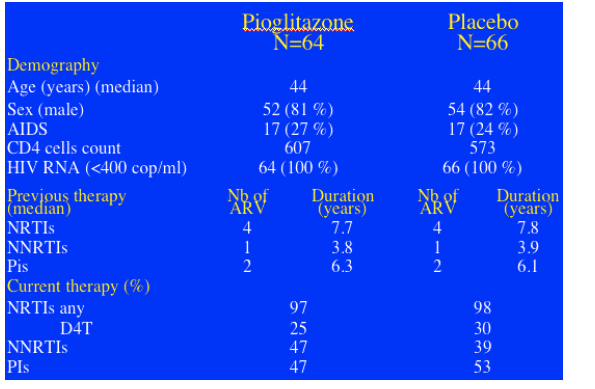
The two groups were well balanced at baseline. The median number of signs of lipoatrophy was 4. Median duration of ART was 7.7 yrs. 89% of patients had been exposed to d4T for a median of 3.2 yrs. At baseline 28% of patients were still on d4T. The 2 groups were well-balanced for body composition with limb fat mass of about 2.9 kg.
BASELINE CHARACTERISTICS
Study patients average age was 44 yrs. 80% were men. 25% had AIDS at some time. Current CD4 counts were 550-600. 64% had <400 c/ml. Duration of NRTI experience was 8 yrs; NNRTIs 4 yrs; PIs 6 yrs. 97% were on NRTIs & 28% on d4T. 50% were on PIs.
NRTI INTERRUPTIONS DURING FOLLOW-UP
5 patients on PIO stopped ARV treatment; 3 stopped d4T. On placebo, 2 switched therapy, 1 interrupted 2 stopped d4T.
|
|
| |
| |
|
 |
 |
|
|