 |
 |
 |
| |
Schering CCR5 Vicriviroc- details on why study in treatment-naives was stopped but new dose is being explored
|
| |
| |
Reported by Jules Levin
"Virologic Breakthrough in treatment-Naive Patients on a Regimen of Combivir + Vicriviroc, Schering's CCR5 Drug"
Wayne Greaves from Schering Plough reported on why this study was stopped and presented the study results in Denver at the 13th CROI (Feb 5-8, 2006). Of note, although the study was stopped Greaves and Schering announced that the company will explore a higher dose as it was felt by the information presented at this meeting that the dose of vicriviroc used in this study was too low and may be the reason for the viral failures causing the study to be stopped. As well, study of this drug continues in treatment-experienced patients in an ongoing ACTG study. First Greaves briefly described the drug. Vicriviroc is a small molecule CCR5 receptor antagonist, which in phase I trials showed potent antiviral activity of about a 1.6 log viral load reduction. The drug is primarily metabolized by the CYP3A4 pathway. It is not a pgp substrate. It has a long halflife of about 27 hours and therefore is dosed as a once a day oral drug. This talk was the first public presentation at a conference reviewing details from the phase II study conducted in 92 treatment-naive patients regarding why it was stopped. Below you will find a detailed review of the information presented. Greaves discussed safety & resistance observed during this study, and most importantly the viral failures or breakthroughs that occurred following the 14-day monotherapy leadin that caused the study to be stopped. This study had been stopped in October of 2005 because of more virologic breakthroughs in the Vicriviroc arm than in the comparison arm. This study was conducted worldwide and in treatment-naive patients with >150 CD4s and 5,000 copies/ml of HIV RNA. Patients had to have no baseline resistance to the ART components used in the study -- efavrirenz, AZT, & 3TC. Treatment-naive was defined as it often is in studies, fewer than 2 weeks prior total ART experience. The study was designed to run for 48 weeks and was designed to have an initial monotherapy phase of 14-days, where there were 3 doses of vicriviroc - 25, 50, and 75 mg once daily compared to placebo. After the two weeks monotherapy Combivir was added to each of the arms and efavirenz was added to the placebo arm along with Combivir. The study design is depicted graphically below. Greaves said the doses chosen were based on the findings in their PK program. Of note, the primary study analysis was conducted when all study subjects completed the two weeks of dosing, it was upon this information that led to study termination.
Greaves ended his talk with these Conclusions:
--vicriviroc was safe & well tolerated: no hepatocellular injury was observed and he stressed that none has been observed in studies. A dose response was seen for both the day-14 and sustained viral responses. HIV RNA change at day 14 was a strong predictor of sustained viral response. Tropism shift was infrequent and seen in all groups, including placebo - most cases occurred during the first two weeks. At the doses used in this study, vicriviroc+Combivir did not provide the same sustained response observed with efavirenz+Combivir. The optimal dose and role of vicriviroc in ART regimens in this patient treatment-population remains to be defined and we are in the process of is currently being explored.
After this presentation it was apparent that perhaps these failures occurred because the doses selected to be studied may be too low. At the doses used in this study, vicriviroc + Combivir did not provide the sustained response observed with efavirenz+Combivir, Greaves said. The good news is that no serious safety concerns associated with vicriviroc have been reported by Schering. Greaves reported in a slide that "no clinically significant changes in labs" occurred, there is "no evidence of hepatocellular injury", there has been "no QTc or other ECG abnormalities, there were "no dose-related AEs". Greaves said in his presentation there were 9 treatment emergent SAEs, but they were all considered unrelated to vicriviroc: and he said vicriviroc was well tolerated. Vicriviroc & the safety and side effects profile were said to be good so higher doses are expected to be explored.
In addition, there was a 2-week monotherapy lead-in period, which also may have played a role in causing the failures in conjunction with too low a dose. There was discussion about the study and several comments were made by observers in the audience during the question & answer period following Greaves' talk. Greaves was asked if it was the tropism shift patients who developed the M184 mutation. In response Greaves said 'not necessarily'; Greaves commented that some of the patients with tropism shifts had M184 mutations but 'it was not an all or none phenomena' and as far as we could follow the patients with tropism shifts they have responded well to other ART regimens subsequently prescribed by their physicians. One comment said it was hard to evaluate the relative potency of this drug vs the EFV-based regimen without looking at the viral load response curves in the 24-week results in the 4 arms of the study & asked to see them, but Greaves said he did not have them here. Greaves said there was only 1 failure in the EFV arm. A second comment was regarding the study design & the 14-day lead-in monotherapy, and it was suggested by the commenter that the design was 'high risk' .... 'considering that you are dealing with envelope and a CCR5 inhibitor', and vicriviroc was not given 'its best opportunity to perform'. Greaves responded by saying at the time of study design they conferred with their experts and had much discussion & also combined with the information they had at that time that resistance was hard to produce taking about 16 weeks of in vitro passaging. Greaves said that at that time they felt the 14-day lead-in was reasonable but in retrospect the monotherapy leadin might have 'given us a different outcome'.
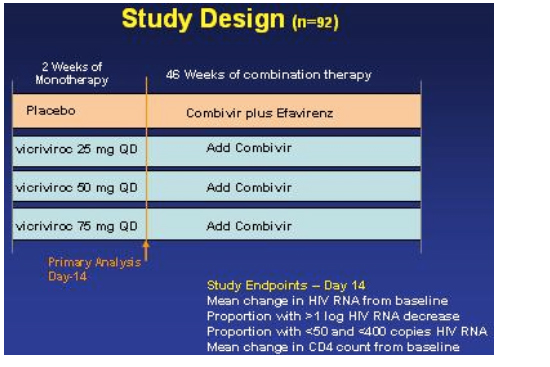
STUDY OBJECTIVES: To evaluate the safety, tolerability, and antiviral activity of vicriviroc:
--plasma HIV RNA measured at screening, baseline, day-4, day-7, day-14, monthly to 6 months, then bimonthy
--tropism tests at screening, baseline, day-14, week-24, and at virologic failure or wk-48
--PK trough vicriviroc concentrations measured at virologic failure or wk-48
--12-lead EKG at screening, day-14, wk-24, and wk-48
The primary analysis was conducted when all study subjects had completed two weeks of dosing.
The study endpoints were:
--mean change in HIV RNA from baseline
--proportion with >1 log HIV RNA decrease
--proportion with <50 & <400 copies/ml
--mean change in CD4 count from baseline
Baseline patient characteristics included: median age: 37 yrs (range: 18-72); 80% men, 14% non-caucasian; median CD4 count of 290 cells (range: 103-687); median HIV RNA of 4.79 log (range: 3.55-6.02); HIV subtype B: 78% of isolates; and Greaves said the characteristics were balanced across arms.
In the 14-day monotherapy phase of the study, the 75 mg dose arm saw a mean -1.34 log reduction in viral load, the 50 mg arm saw a mean reduction of -1.18 log, and the 25 mg dose group saw a viral load reduction of a mean -0.99 log. Greaves said, "all through groups had viral load reductions statistically signicantly better than placebo; there was a statistically significant difference between the lowest dose of 25 mg and the highest dose of 75 mg.
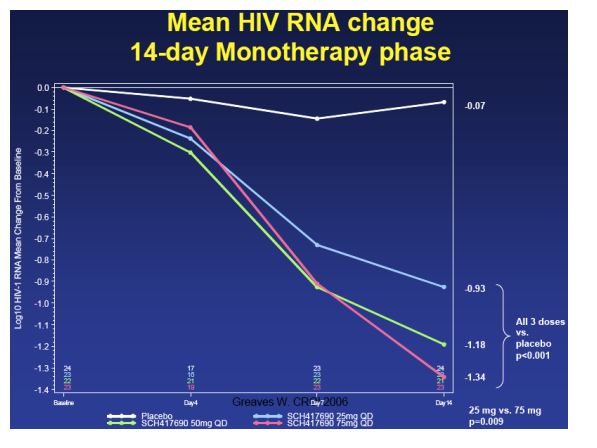
Viral breakthrough >50 copies/ml) by treatment arm at Study Termination
Again, the study was terminated in October 2005 and the reason for termination was the finding of the number of viral breakthroughs in the vicriviroc arm compared placebo. This table immediately below shows the relative rates of virologic breakthroughs in the vicriviroc arms compared to the pacebo arm. As you can see in the table, 56% (13/23) of the patients in the 25 mg dose arm experienced viral breakthrough (vs control, p<0.001), 41% (9/22) in the 50 mg arm (vs control, p=0.003), and in the 75 mg arm 17% (4/23) experienced viral breakthrough (vs control, p=0.188). At the time of study termination, there was no significance difference in viral failure breakthrough between the 75 mg arm & the EFV/CBV arm.
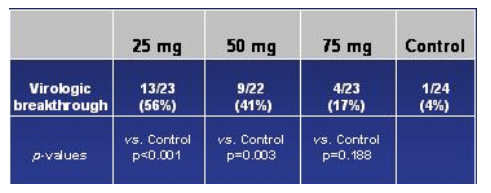
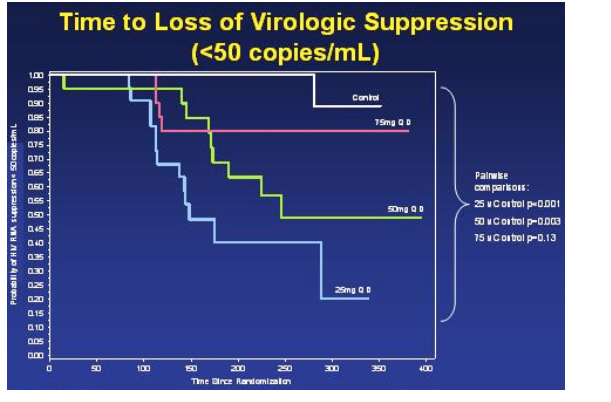
Kaplan-Meier Estimate: Time To Loss of Virologic Suppression
This slide immediately below, the Kaplan-Meier Estimate depicts much of the same information as the slide on the relative risks of viral breakthroughs, but shows the Time To Loss of Viral Suppression (>50 copies/ml). Greaves commented that the protocol was written to use 400 copies as the cutoff but the DSMB used 50 copies. Again, this graph shows there was a statistically significant difference between the 25 mg arm vs control (p<0.001), and between the 50 mg arm vs control (p=0.003). However, again there was no statistically significant difference in time to loss of viral suppression between the 75 mg arm & the control arm (p=0.13). If you pool all the vicriviroc arms & compare to the control arm, there is a statistically significant difference. Also, if you pool the 50 & 75 mg arm & compare it to the control arm, there is a statistically significant difference.
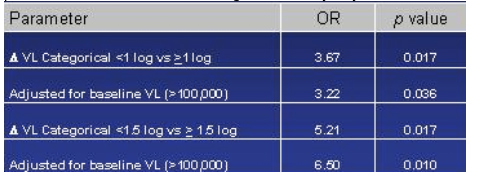
Day-14 Viral Load reduction as a predictor of sustained virologic suppression: reduction in viral load from baseline to day 14 was a predictor of failure.
This table depicts an analysis they did to identify any baseline characteristics that could predict viral suppression or failure. The only one that stood out was the viral load reduction from baseline to day 14. They chose two cutoffs to look at: 1 log reduction & 1.5 log reduction. The odds ratio for viral failure if a patient did not achieve a 1.5 log reduction by day 14 was 5.21, If this was adjusted for baseline viral load >100,000 the odds ratio was even higher, 6.50 for those patients not able to reach a 1.5 log reduction by day 14.
STUDY DESIGN
Study patients were randomized to weeks of monotherapy of: placebo, vicriviroc 25 mg once daily, vicriviroc 50 mg once daily, or vicriviroc 75 mg once daily. After 2 weeks Combivir was added to the vicriviroc arms and placebo patients receiced efavirenz + Combivir.
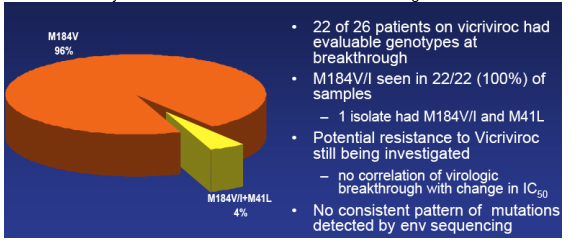
Resistance: Subjects with virologic Breakthrough; M184V/I+M41L (4%); M184V (96%)
Greaves said resistance is something they continue to look at but here are some points important to be made, depicted in this table. 22 of 26 patients on vicriviroc had evaluable genotypes at breakthrough. M184V/I, the 3TC/FTC mutation, was seen in 22/22 (100%) of these patient's isolates. One isolate had the M41L in addition to M184V/I. Greaves said, they are still trying to understand vicriviroc resistance; they have found no correlation between viral breakthrough with baseline mic values or with change in IC50s at day 14 or at the time of viral failure; they have also looked at percent of maximal suppression and IC half max and we've looked at these values at baseline and the change at day 14 and they find no correlation between those values and viral breakthrough; they still haven't completed looking at resistance patterns & there is 'still work to be done' looking at the percent of maximal suppression change at the time of viral failure, and they are aware of data presented at this CROI suggesting that flattening of the IC50 curves may be correlated with to resistance to these agents.
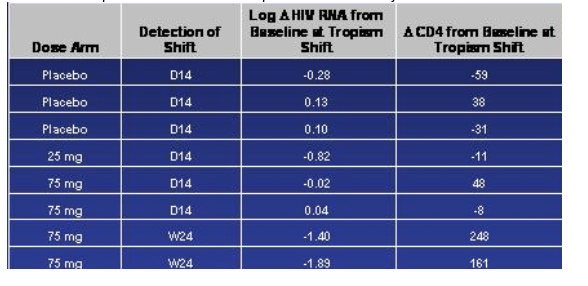
Treatment-emergent Tropism Shifts Occurred But Infrequently
They have looked at only a few envelope sequences so far, Greaves said. Of the 13 they have looked at, they haven't found any unique mutations or patterns of mutations that correlate with viral breakthrough. But this work is still ongoing. Greaves said tropism shift was 'all the buzz and the rave' when these studies were started. They did see tropism shifts but it was infrequent. It is perhaps more important, Greaves said, that tropism shifts were seen not only in the vicriviroc arms but in placebo. There were 8 tropism shifts that they noted that occurred during the course of treatment, 3 in the placebo arm, 1 in the 25 mg arm, and 4 in the 75 mg arm. Greaves said, note that 6 of the 8 shifts occurred relatively early, within the first 14 days, and in fact one patient had a change by day 4. And one may reasonably argue, Greaves said, these represent minority variants that emerge with the use of R5 suppression; we haven't however confirmed this yet with clonal analysis. Greaves said I think we can take some comfort from the two patients in the 75 mg arm where this shift was detected at week 24; and also point out that because we did tropism shift check at day 14 and not again until week 24 unless the patient patient failed it doesn't necessarily mean that shifts occurred at week 24; the point to be made here is that there is some preservation in antiviral response and the CD4 response in these subjects.
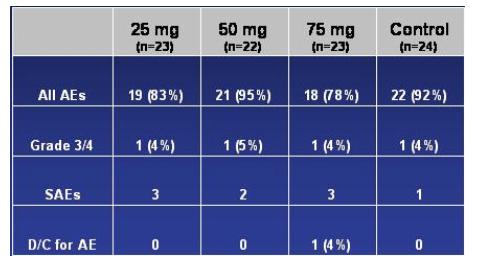
Overall Safety Results
The table below is what was presented regarding safety in the presentation.
No clinically significant changes in labs: no evidence of hepatocellular injury and Greaves stressed that there has been so far no evidence of hepatocelluar injury in studies with vicriviroc. There were 4 grade 3-4 AEs, one in each of the 4 study arms. There was no mention of the specific adverse events observed. Greaves referred to one patient who discontinued study due to AZT-related toxicities.
No QTc or other ECG abnormalities
No dose related AEs: 9 treatment-emergent SAEs, all considered unrelated to vicriviroc
--Vicriviroc was well-tolerated overall
|
|
| |
| |
|
 |
 |
|
|