 |
 |
 |
| |
Treatment Interruptions (STIs)
|
| |
| |
Written by Mike Youle, MD
Royal Free Hospital, London, UK
Stop-start, stop-start, like a pushme-pullyou, no one seems quite sure of the value or otherwise of treatment interruptions. However this conference gave the best selection so far presented of structured treatment interruption studies. Jim Neaton from the SMART study team rather pompously seemed to think that being the biggest study this was the definitive one and that everyone should agree they were a bad idea. However it was clear from the very different study designs and, varied stopping rules and endpoints that this is far from a simple issue. Below is a comparison of the 6 studies oral presentations at the meeting, plus the abstracts of each study; I have inserted a few slides form each presentation I had access to.
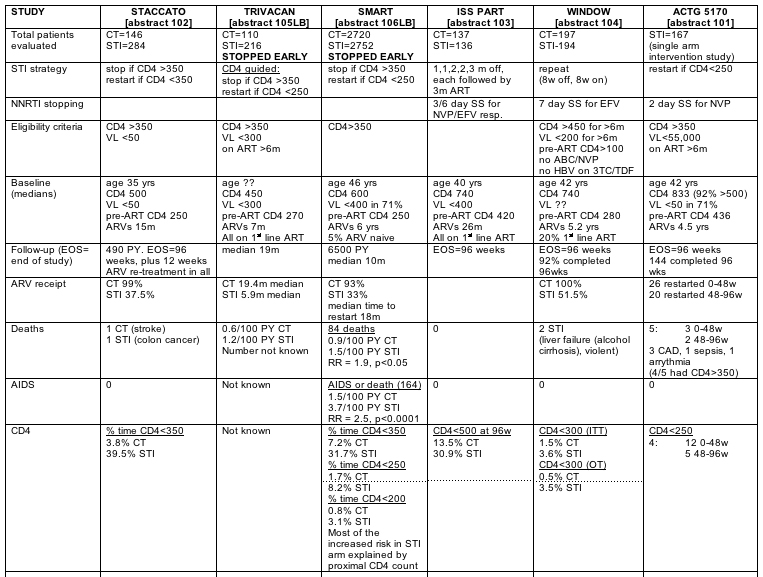
Other notes:
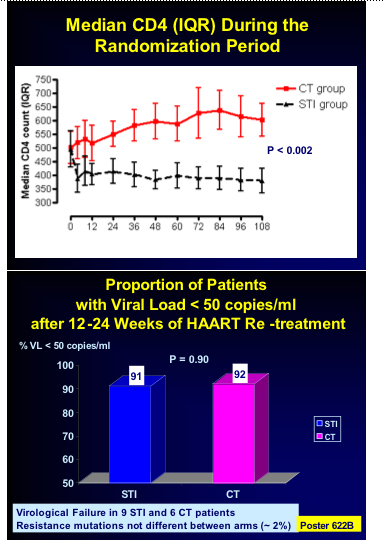
STACCATO presentation noted that incidence rates in SMART would have produced -17 AIDS defining illnesses or death in Staccato's STI group, whereas no AIDS defining illnesses and only 1 death occurred.
TRIVACAN also has a fixed STI arm (2m off, 4m on, N-300) which is continuing to end of study together with the CT arm. Results not presented.
The main trials:
Abstract 101:
Predictors of HIV Disease Progression in Patients Who Stop ART with CD4 Cell Counts >350 cells/mm3 ACTG 5170
Daniel Skiest, Baystate Med Ctr, Springfield, MA, US
Methods: ACTG 5170 was a multicenter, prospective study of HIV-infected patients with CD4 cell count >350 cells/mm3 prior to ART and within 45 days of study entry, HIV RNA viral load <55,000 copies/mL within 45 days of study entry, and on ART (≥2 drugs) for ≥6 months. Subjects stopped ART at entry and were followed for 96 weeks. ART was restarted at the discretion of the patient and health care provider. The primary endpoint was time to CDC Category B or C event or death or CD4 count ≦250 cells/mm3.
Results: We enrolled 167 subjects. The median entry CD4 count was 833 cells/mm3 and 136 subjects had viral load ≦400 copies/mL. The median nadir CD4 count was 436 cells/mm3 (IQR 375 to 510). The median time on ART was 3.9 years and the median number of cumulative adverse reactions was 3 (range, 3 to 9). The median length of ART interruption was 96 weeks. CD4 cell decline following ART cessation occurred in 2 phases: 198 cells/mm3 in the first 8 weeks; then 1.74 cells/mm3 per week, from week 8 through 96. The median time to reaching viral load set-point off ART was 4.3 weeks (IQR 3.1 to 8.4 wks). Following ART cessation, 1 subject had the acute retroviral rebound syndrome and 4 subjects had thrombocytopenia (platelet count <55,000). A possible or definite CDC category B or C diagnosis occurred in 3 and 2 subjects, respectively (all with CD4 >350 cells/mm3). At 96 weeks, 17 patients had confirmed CD4 count ≦250 cells/mm3 and 46 subjects had restarted ART. During the study, 5 subjects died, but no death was related to HIV/AIDS diagnoses. Of 28 subjects, 24 with viral load ≦400 copies/mL at entry, and who restarted ART achieved a viral load of ≦400 copies/mL. In univariate analysis, shorter time to primary endpoint at week 48 and 96 was predicted by low CD4 count nadir, lower entry CD4 count entry, and higher entry viral load. In multivariate analysis only CD4 nadir was predictive (p = 0.049). Activated CD4 or CD8 phenotype at entry correlated with greater CD4 decline at week 96 (p <0.05).
Conclusions: ART interruption was generally safe in this cohort. Low nadir CD4 count is the best predictor of CD4 decline or clinical events following ART cessation.

Abstract 102:
CD4-guided Scheduled Treatments Interruptions Compared to Continuous Therapy: Results of the Staccato Trial
Jintanat Ananworanich, HIV Netherlands Australia Thailand Res Collaboration, Bangkok, Thailand
Methods: We randomized 430 patients with CD4 counts >350, and viral load <50 1:2 to either continuous therapy (n = 146) or scheduled treatment interruption (STI) (n = 284), with treatment stops as long as CD4 counts exceeded 350. The median time on randomized treatment was 21.9 months. At the end of the study, both groups were again treated continuously for 12 to 24 weeks. In Thailand, 352 patients received 1600 mg of saquinavir (SQV) with 100 mg of ritonavir (RTV) once daily with 2 nucleoside reverse transcription inhibitors (NRTI): didanosine/stavudine (ddI/d4T) from 2002 until March 2003, and tenofovir/lamivudine (TDF/3TC) after that. In Swiss and Australian patients, the type of HAART used was defined by the treating physician.
Results: The probability of restarting treatment in STI was 53% at 6 months, 64% at 12 months, and 74% at 24 months. Drug savings in STI, compared to continuous therapy, amounted to 61.2% during randomized treatment. In an intent-to-treat analysis, the percentage with viral <50 copies was 91.8 % in continuous therapy, compared to 90.3% in STI after re-treatment at the end of the trial (delta 1.6%, 95%CI –4.0 to 7.3%). At the end of randomized treatment, median CD4 counts were 374 in STI (60.5% >350), and 601 in continuous therapy (96.2% >350, p <0.002). After re-treatment, median CD4 counts rose in STI from 374 to 459 after 12 weeks, with 85.9% >350, compared with 96.9% in continuous therapy (p <0.01). During STI, 17 patients (5.8%) had symptoms of acute retroviral syndrome. Diarrhoea (p = 0.04) and neuropathy (p = 0.03) were more frequent in continuous therapy, whereas oral and vulvo-vaginal candidiasis (p = 0.03) and thrombocytopenia (p = 0.06) were more frequent in STI. Sequencing was attempted in 125 patients where resistance was most likely because of numerous stop-start cycles, problems with compliance, and/or viral breakthrough. We observed no resistance mutations in the RT and P genes in 115. Resistance mutations occurred in 7 patients in the RT gene, and 3 in the P gene.
Conclusions: During 484 patient-years of STI, little evidence of treatment resistance emerged. Treatment-related adverse effects were more frequent in continuous therapy, but minor manifestations of HIV infection were more frequent in STI.
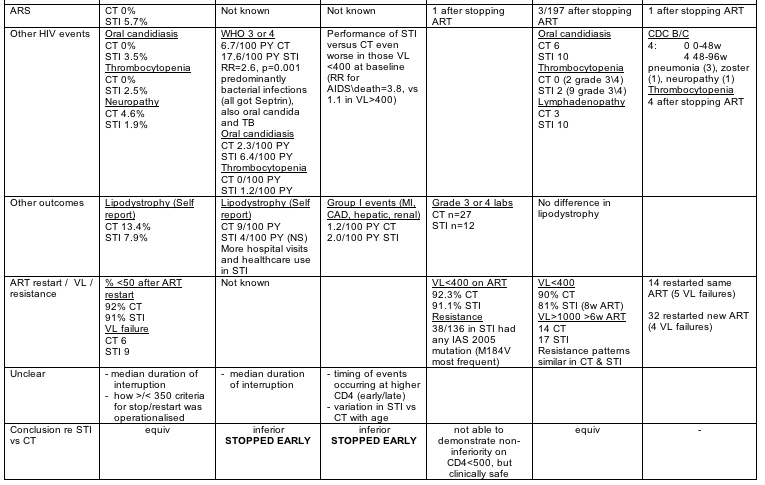
Abstract 103:
Final Results of a Randomized, Controlled Trial of Structured Treatment Interruptions vs Continuous HAART in Chronic HIV-infected Subjects with Persistent Suppression of Viral Replication; ISS PART
Lucia Palmisano, Clin Ctrs Inst Superiore di Sanità, Rome, Italy
Methods: Were enrolled 273 subjects, 136 in arm B (5 structured treatment interruptions of 1-, 1-, 2-, 2-, and 3-month duration, each followed by 3 months of therapy) and 137 in arm A (continuous HAART). Primary end-point was the proportion of patients with >500 CD4/mm3 after 24 months. Mutations in proviral DNA and residual replication (assay cut-off: 2.5 copies HIV RNA/mL) were measured at baseline. Genotypic resistance was tested during structured treatment interruptions. Both per-protocol and intention-to-treat analyses were conducted.
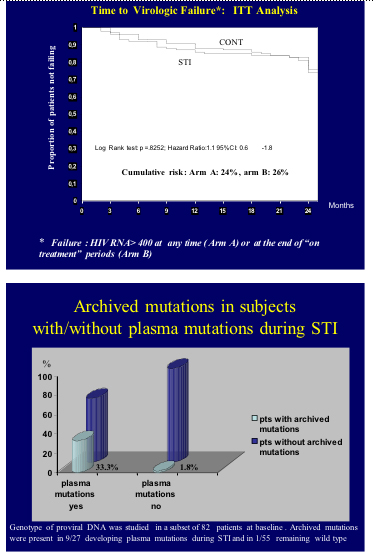
Results: Risk of protocol discontinuation was higher in arm B (66.5% vs 19.4% in A, hazard ratio = 4.6, 95%CI 3.0 to 7.3); conversely, more patients achieved primary endpoint in arm A (86.5 % vs 69.1% in B in the per-protocol analysis, p = 0.0075). Probability to complete study protocol and achieve the primary endpoint in arm B were independently predicted by pre-HAART CD4+ and male sex. Similar rates of virological failure were observed in A and B (24% and 26%, respectively). However, in arm B 2 factors entailed a higher risk of failure: baseline residual replication >2.5 copies/mL (30.4% virological failure vs 11.3% in those with <2.5 copies/mL, HR =2.9, 95%CI 1.1 to 7.5) and plasma mutations during structured treatment interruptions. Indeed, of 136 subjects, 40 (29.4%) developed mutations in arm B, corresponding to a 32% (95%CI 23.5 to 40.0) cumulative risk over 24 months. Of them, 13 (32.5%) failed to achieve HIV viremia values <400 copies on ≥1 occasion at treatment resumption, compared with 14 of 96 (14.6%) patients without mutations. In a time-dependent Cox regression model this yielded a higher risk of virological failure associated with resistance (HR 2.3, 95%CI 1.0 to 5.1). The emergence of plasma mutations was independently predicted by the presence of archived mutations in proviral DNA at baseline and the use of a regimen based on unboosted protease inhibitor (PI) in a multivariate model (p = 0.001 for DNA mutations and p= 0.040 for PI-HAART).
Conclusions: Potential candidates to structured treatment interruptions are subjects with high pre-HAART CD4 count, absence of archived mutations, and residual replication <2.5 copies HIV RNA/mL. For this therapeutic strategy NNRTI-based regimens appear preferable to unboosted PI HAART.
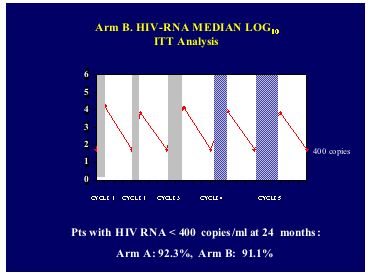
Abstract 104: Structured Treatment Interruptions in HIV-infected Patients with High CD4 Cell Counts and Virologic Suppression: Results of a Prospective, Randomized, Open-label Trial (Window - ANRS 106)
Bruno Marchou, Hosp Purpan, Toulouse, France
Methods: We randomly assigned to 2 groups 403 adults with a nadir CD4 >100/mL, plasma HIV RNA viral load <200 copies/mL, and CD4 ≥450/mL for ≥6 months while on HAART. One group switched to an 8-weeks-off/8-weeks-on intermittent therapy; the other maintained continuous therapy for 96 weeks. The primary outcome was the cumulative incidence of immunologic failures (confirmed CD4 <300/mL). Non-inferiority of the intermittent vs continuous therapy arm was obtained if the upper bound of the 95% confidence interval of the difference (UBCID) in proportion of immunologic failures remained L7%.
Results: Of 403 patients, 12 withdrew consent. At baseline, 80% of patients were male, with a median age of 41.6 years, and 8% had AIDS. Median CD4 was 741/mL with a nadir at 280/mL; 43% and 46% of patients were on a non-nucleoside reverse transcriptase inhibitor (NNRTI)- and protease inhibitor (PI)-based regimen, respectively; 7 (4%) and 12 (6%) patients in the intermittent and continuous arm were lost to follow-up. In the intermittent are, 2 patients died (deaths were not related to HIV). No AIDS-defining event was recorded. Median (IQR) proportion of time on therapy, intermittent vs continuous, was 51.5% (49.5, 55.7) and 100% (100, 100). The proportion who reached the primary immunologic endpoint was: in intent-to-treat analysis 7 (3.6%) vs 3 (1.5%) (UBCID: 5.6%); in per patient analysis 6 (3.5%) vs 1 (0.6%) (UBCID: 6.5%). All these patients had a nadir CD4 <300/mL. At week 96, the proportion of patients, intermittent vs continuous, with CD4 >450 /mL and with viral load ≦400 copies/mL was 75% vs 92% (p <0.0001) and 81% vs 90% (p = 0.02). The first 200 patients were enrolled in a virologic sub-study. The proportions of patients with a viral load ≥1000 copies/mL after ≥6 weeks of therapy (defined as virologic failure) were similar in the intermittent therapy (17 of 98) and continuous therapy arms (14of 99, Log rank p = 0.51). Resistance genotype available for 24 patients (14 intermittent therapy, 10 continuous therapy) was wild type in 9 (7, 2, respectively) and with main resistance mutations in 15 patients (7, 8, respectively). Median number of mutations per patient was 4 in the intermittent therapy and 5 in the continuous therapy arm (p = 0.07). Resistance mutations to protease inhibitors were detected in only 3 patients (1, 2, respectively), to NRTI in 13 patients (7, 6, respectively), and to NNRTI in 7 patients (1, 6, respectively).
Conclusions: In this population of patients, a fixed structured treatment interruption strategy of 8-weeks-off/8-weeks-on appeared clinically and immunologically safe over 96 weeks while sparing 48.5% of drug exposure. Patterns of drug resistance mutations in patients with virologic failure appeared similar.
Abstract 105LB:
The CD4-guided Strategy Arm Stopped in a Randomized Structured Treatment Interruption Trial in West-African Adults: ANRS 1269 Trivacan Trial
Christine Danel, Ctr Hosp Univ de Treichville, Abidjan, Cote d'Ivoire, France
Methods: Patients on suppressive HAART with CD4 >350/mm3 and plasma viral load <300 copies/mL were randomized to receive either: continuous therapy (CT) (1 of 6 patients); or CD4-guided therapy (CGT) with introduction/interruption thresholds of 250/350 CD4/mm3 (2 of 6 patients); or 2-month-off/4-month-on therapy (2MO/4MOT) (3 of 6 patients). The endpoints were death and serious morbidity (any WHO stage 3 or 4 classifying event). An interim analysis was reviewed by an independent Data Safety Monitoring Board (DSMB) in October 2005. The DSMB recommended continuing the CT and 2MO/4MOT arms until trial termination, and stopping the CGT arm prematurely. We report only data from the CT and CGT arms.
Results: We randomized 110 patients in the CT and 216 in the CGT arms. The main baseline characteristics (all non-significantly different between arms) were: 77% women; 95% infected with HIV-1 alone; median age 34 years; median CD4 460/mm3; HAART regimen: 91% 2 NRTI + 1 NNRTI, 9% 2 NRTI + 1 PI. By October 31, 2005, overall median follow-up was 20 months, 4 patients were lost to follow-up, 5 patients were dead (incidence 0.6/100 person-years in CT arm, 1.2/100 person-years in the CGT arm, relative risk [RR] 0.48, 95%CI 0.01 to 4.91), and 60 patients had experienced 84 serious morbidity events (CT 6.7/100 person-years, CGT 15.2/100 person-years, RR 0.44, 95%CI 0.21 to 0.87). These clinical events were 42 oro-pharyngeal candidiasis (CT 2.3/100 person-years, CGT 6.4/100 person-years, RR 0.36, 95%CI 0.09 to 1.08), 24 invasive bacterial events (CT 0.6/100 person-years, CGT 6.7/100 person-years, RR 0.08, 95%CI 0.01 to 0.52), 16 tuberculosis (CT 2.3/100 person-years, CGT 3.6/100 person-years, RR 0.65, 95%CI 0.15 to 2.14), and 2 others. Of the 24 bacterial events, 14 occurred with documented bacteraemia (all in the CGT arm) and 10 occurred without bacteraemia but with hospital admission. In the 286 patients who reached month 12 after randomization (CT 96, CGT 190), the median (interquartile range [IQR]) month 12 CD4 count was 546/mm3 (IQR 452 to 645) in the CT arm and 333/mm3 (IQR 286 to 404) in the CGT arm.
Conclusions: CGT led to a 2-fold higher serious morbidity rate than CT, mainly due to invasive bacterial diseases. Further structured treatment interruption trials assessing CGT strategies in sub-Saharan Africa should use higher CD4 thresholds for interruption/introduction. Whether a fixed treatment-interruption strategy is appropriate in Cote d'Ivoire requires further follow-up.
Abstract 106LB:
Episodic CD4-Guided Use of ART Is Inferior to Continuous Therapy: Results of the SMART Study
Wafaa El-Sadr, New York, USA
Methods: Patients with CD4 >350 cells/mm3 were randomized to a CD4-guided drug conservation (DC) arm or to continuous ART aimed at viral suppression (VS arm). In the DC arm, ART was stopped until the CD4 count fell to <250 cells/mm3, when ART was initiated; ART was stopped again when CD4 >350 cells/mm3. The primary endpoint was HIV disease progression or death. To detect a 17% difference between arms, 6000 patients were required. On 10 January 2006, the DSMB notified the team of increased HIV progression risk in the DC arm. Enrolment was stopped on January 11.
Results: A total of 5472 patients were enrolled from 318 sites in 33 countries. The majority (95%) were ART experienced (median 6 years prior ART use). The mean age was 46 years; 26% were women and 31% black, 69% white or other. At baseline, median CD4 was 598 cells/mm3, nadir CD4 was 253 cells/mm3, 70% had HIV RNA <400 and 24% prior AIDS diagnosis. By December 10, 2005, median follow-up was 10 months (IQR 4 to 23), with 6139 person-years of follow-up; 2.1% of patients were lost to follow-up. In the DC and VS arms, ART was used 33% and 93% of the follow-up time and patients spent 938 (31.7%) and 236 (8.1%) patient-years at CD4 <350 cells/mm3, 276 (9.3%) and 59 (2.0%) patient-years at CD4 <250 cells/mm3, respectively. Confirmed clinical endpoints are shown in the table. The relative risk of disease progression or death (DC/VS) was greater in the first 2 years of follow-up (RR = 2.7, 95%CI 1.8 to 4.2) compared to afterward (RR = 1.1, 95%CI 0.6 to 2.2).
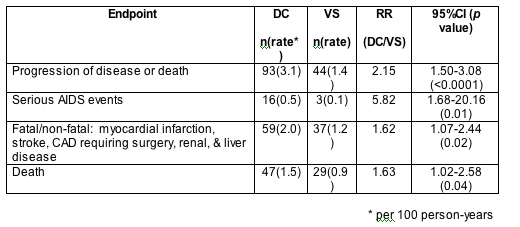
Conclusions: SMART, a large international trial, demonstrated that CD4-guided episodic ART use aimed at maintaining CD4 >250 is associated with increased short-term risk of progression of HIV disease and death compared to continuous ART.
After the presentation of the studies there was an in promptu panel discussion with the following Participants:
- Diane Havlir; San, Diego USA [DH]
- Paul Volberding; San Francisco, USA [PV]
- Patrick Yeni; Paris, France [PY]
- Bernard Hirschel ; Geneva, Switzerland [BH]
- Sharon Walmsley; Toronto, Canada [SW]
- Waafr El-Sadr; New York, USA [WE]
- Stefano Vella; Rome, Italy [SV]
John Bartlett chaired the session and first asked each participant what the main issues were.
- Vella said three issues; firstly different studies had very different designs and that clearly the T4 threshold for restarting was important and probably too low at 250; second that STI has been seen as a harm reduction strategy and at least SMART had shown that to not be true and thirdly that it was important to define a strict resistance definition for use in these studies since mutations seen after STI may be new or may represent archived mutations.
- El-Sadr said that a lot had been learned through these studies and that is was vital to put the efficacy toxicity balance in context. She was surprised that drug conservation (in SMART) was not protective of serious events and said that we had learned a lot about the risks of disease progression in patients off treatment which could be different from treatment naïve individuals.
- Walmsley said that the studies opened the door to many more questions but that it was important to state that there was no influence on the time of starting therapy and that no guideline change was necessary with respect to this. She was worried that end-point biases could have crept in (e.g. candidiasis) since all the studies were open design and was curious as to whether the rise in cardiovascular disease seen could be due, in part, to inflammatory cytokines. One goal had been to reduce cost and that did happen although cost-effectiveness data was also required.
- Hirschel noted that the duration of interruption seemed to be important and that the Kaplan Meier plots of the large studies suggest that divergence between two groups only really occurred after six months off therapy. He stated that the studies that had shorter STI's and more frequent follow-up seemed not to be showing major differences and I have to say this was my major concern about the study as we appeared to be following patients less closely that we currently do in clinical practice.
- Havlir felt that each study taught us something and that they ranged from very dangerous to relatively safe. In addition it had been disappointing that no new markers or predictive factors for progression had emerged; finally that cardiovascular disease was very important and that the issues of pregnancy and co-infection, especially of HBV, had to be taken into account when planning an STI.
- Yeni seemed to think that the main message was that the study design and patient population combined with stopping rules seemed to determine the various outcomes and that what emerged as the successful approach was to have the T4 maintained within the normal range throughout the study and to limit the off therapy time.
Then came questions from the floor and the first from Andy Zolopa to Waafr El Sadr asking what the major endpoints in SMART were if they were not AIDS events to which she replied those related to HCV, cardio vascular disease and violence. At this point Vella suggested that what was needed was a further SMART study with different T4 thresholds, a position supported by Yeni. Hirschel noted that the issue of stopping therapy would not go away and that SMART on face value suggests that when you start you cannot stop, which only increases the importance of undertaking randomised START studies, however difficult they may be to do.
Pablo Tebas asked in view of the results shown how IRBs and investigators should deal with the many ongoing studies where treatment interruption is part of the design such as vaccine studies. Havlir noted that at this time it is completely reasonable to continue such trials after discussion with IRB.
Dominique Costagliola from France asked if in SMART there was any relationship between the number of stops and the risk of disease progression and was told that at the present no.
Hirschel said that one unanswered question was what would happen over the much longer term, bearing in mind that the follow-up of most of these studies was relatively short. Stepwise decrease in median T4 levels was seen in several trials notable WINDOW and it was unclear if this would happen in all studies and at what rate.
The panel was then asked what they thought the questions to be answered were:
- Yeni thought it was necessary to refine STI studies to answer specific important questions such as resistance and adverse event rates which may be possible initially in Pilot studies. Taking into account that drug toxicity in general is declining the cost issue is probably becoming more of the reason for STI's
- Havlir agreed and suggested that PK and genetics studies also would be of increasing importance and that clearly defining restart rules was vital.
- Hirschel suggested that SMART opened the window on pathogenesis since there had been an assumption that most liver and CVD was due to treatment rather than disease, he thought that more side effects off drug was puzzling.
- Walmsley wanted to understand more about the immediate stopping period and the role of cytokine release and immune perturbation around this time.
- El-Sadr was interested in quality of life and metabolic evaluation since any future studies since these may also be the drivers of real life outcomes rather than point events within a study.
- Vella noted that there was an ongoing Paediatric study within the PENTA network and that the new NEAT network to undertake large studies in Europe could co-ordinate future studies effectively. He thought that the tight banding of T4 in most of these trials between 250 and 350 was not helpful; "give some breath to thresholds".
At this point there were less than 200 delegates left in the audience which considering the importance of the topic seemed rather too few.
There have been two papers recently published on treatment interruptions in AIDS which have relevance to this discussion. The first by Keh and the group of Cliff Lane in the US looked at the immunotherapy agent interleukin-2 and the utility of this after stopping therapy. This builds on the IL-2 study as an alternative to therapy presented almost 5 years ago (Youle et al) and the TILT study which is nearing completion which randomises individuals to continue HAART, stop HAART or stop HAART and use IL-2 to maintain T4 cell levels. These studies suggest that there are possibilities to reduce immune deterioration when individuals are not on therapy and open the door for further evaluations to help the HAART-sparing approach.
The second published study by Yves Levy and the ANRS looks at the other side of the equation and showed that that therapeutic immunisation, in this case with ALVAC 1433, HIV-LIPO 6T and IL-2, can significantly reduce viral replication when antiretroviral therapy is ceased and allowed for up to a 40% decrease in exposure to antiretroviral drugs over the 15 months of follow-up.
In my view the era of attraction to treatment interruptions has waned since regimens are now safer and easier to take. I believe that future studies are still warranted and that a blind acceptance that the SMART study gives us all the answers is unjustified; it merely opens the door for further studies to evaluate these strategies.
Keh C et al. Interruption of antiretroviral therapy blunts but does not abrogate CD4 T cell responses to interleukin-2 administration in HIV patients.
AIDS 2006, 20:361-369
Levy Y and the ANRS 093 Study Group. Sustained control of viraemia following therapeutic immunisation in chronically HIV-1 infected individuals.
AIDS 2006, 20:405-413
Youle M et al. Randomised study of intermittent sub-cutaneous interleukin-2 (IL-2) therapy without antiretrovirals versus no treatment. XIII IAS Conference. Durban July 2000 [abstract B027]
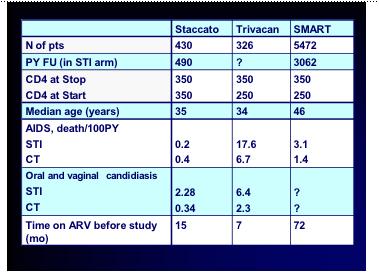
Thank you for those investigators who shared slides from their presentations and specifically Abdel Babiker from the UK MRC for the permission to copy his excellent comparison table.
Mike Youle
|
|
| |
| |
|
 |
 |
|
|