 |
 |
 |
| |
Early, Uninterrupted ART Is Associated with Improved Outcomes and Fewer Toxicities in the HIV Outpatient Study (HOPS)
|
| |
| |
Reported by Jules Levin
Kenneth Lichtenstein*1, C Armon2, K Buchacz3, A Moorman3, K Wood2, J Brooks3, and HIV Outpatient Study
1Univ of Colorado Hlth Sci Ctr, Denver, US; 2Cerner Corp, Vienna, VA, US; and 3CDC, Atlanta, GA, US
This poster presented at the 13th CROI (Feb, 2006, Denver) by Ken Lichtenstein has received quite a bit of attention from the press and in conference coverage by on the internet and by CME providers. This study is retrospective and has limitations, but its findings suggest that certain toxicities or outcomes may be more likely to occur when initiating HAART is delayed past a certain CD4 threshold. The study also finds that when patients were less adherent renal insufficiency & neuropathy occurred more often. The data in Table 1 found diabetes associated with renal insufficiency and lipoatrophy; and hypertension associated with renal insufficiency. I spoke with Dr Lichtenstein and reviewed his poster with him. This report contains the information presented by him in the poster. You will see the data in the tables & graphs report --
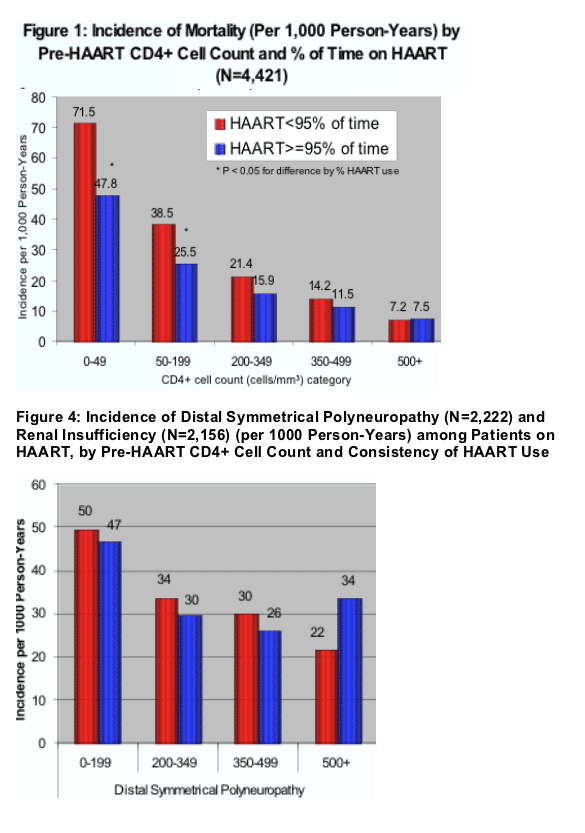
(1) the incidence of mortality (per 1,000 person-years) decreases as the CD4 count HAART was started increases and also increases when the % of time on HAART was less (<95% adherence) (Figure 1): the lowest incidence of moratlity was (HAART adherence <95%/HAART adherence >/=95%; (p<0.05 for difference by % HAART use):
At each level of CD4 count mortality was worse when HAART was started at a lower CD4 count --
- 7.2/7.5 when HAART started at 500+ Cd4 count;
- 14.2/11.5 at 350-499 CD4s
- 21.4/15.9 at 200-349 CD4 count
- 38.5/25.5 at 50-199 CD4 count
- 71.5/47.8 at 0-49 CD4 count
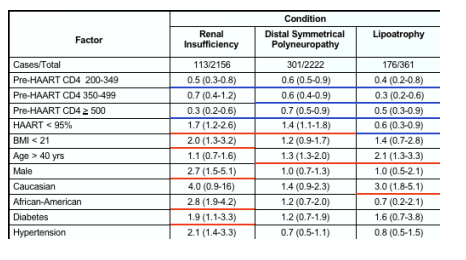
(2) incidence of opportunistic infections (per 1,000 person-years)
increased by pre-HAART CD4 count & % of time on HAART:
-- when CD4 count when HAART was started was 500+: 7.3/2.4
-- 350-499 CD4 count: 16.2/5.4
-- 200-349 Cd4 count: 20.1/10.4
-- 50-199 CD4 count: 37.8/22.3
-- 0-49 CD4 count: 55.9/26.1
Of note, was uninterrupted HAART in his study as well as the results of interruptions in the SMART Study point to a similar concern: HIV causes inflammation & HAATT suppresses inflammation. Treatment interruptions in SMART were associated with unexpected increased renal and cardiovascular toxicities. Stopping HAART may cause the return of inflammation in certain patients.
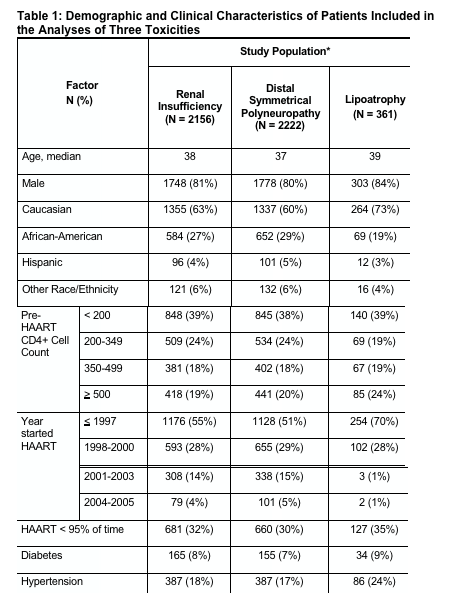
Of interest, in Table 1. Baseline Characteristics, 8% of HOPS study patients had diabetes and 17-24% had hypertension.
As well, in the part of the poster discussion that says that the toxicities may be related to the patient's immune system, this applies to the higher state of inflammation that occurs with more advanced HIV disease. The inflammation is thought to increase the intracellular levels of the nucleoside analogues resulting in greater toxicity in people with lower CD4 counts. It may also explain why people who interrupt therapy have more toxicities since the interruptions result in increased inflammation so the restart of the medications results in higher intracellular drug levels; whereas those who stay on therapy keep the virus suppressed, the inflammation to a minimum, and allow the intracellular levels to reach therapeutic rather than toxic levels.
Statement from the CDC regarding this study:
Beginning highly active anti-retroviral therapy (HAART) early in the course of a patient’s HIV infection may significantly reduce the occurrence of major treatment-related side effects, according to a study conducted by the University of Colorado Health Sciences Center in collaboration with the Centers for Disease Control and Prevention (CDC). The findings add to a growing body of evidence indicating benefits of beginning HAART at higher CD4 counts.
While there has been scientific consensus for may years that HAART should be initiated when a patient’s CD4 count drops below 200 cells/mm3, there has been some debate about the benefits verses risks of initiating treatment at higher CD4r levels. Observational studies have documented that initiating treatment earlier can delay HIV disease progression, but guidelines have stressed the need to balance these potential benefits against concerns about patient adherence and the possibility of greater toxicities.
This study, led by Dr. Kenneth Lichtenstein at the University of Colorado provides new evidence that, in addition to reducing the incidence of HIV-related death and disease, earlier treatment initiation also reduces the risk of adverse side effects associated with therapy. While the reasons for reduced toxicity are not yet fully understood, researchers believe this may be related to the relationship between a patient’s immune function and drug metabolism.
Lichtenstein and colleagues examined the medical records of 2,304 HIV-positive patients in eight U.S. cities who were part of the HIV Outpatient Study (HOPS) between 1996 and 2005. Their analysis compared the incidence of three common toxicities in patients who began treatment at various CD4 levels to the incidence in patients who began at a CD4 count of 200 cells/mm3 or below. Results indicate that patients who initiated treatment at CD4 counts above 350 were at least 60% less likely to develop renal insufficiency, at least 30% less likely to develop peripheral neuropathy, and 60% less likely to develop lipoatrophy than patients who initiated therapy at a CD4 count of 200 cells/mm3 or below.
The findings also suggest that maintaining therapy at least 95% of the time may reduce a patient’s risk of developing these toxicities, as intermittent use was associated with an increased risk of both renal insufficiency and peripheral neuropathy. Study authors believe the findings demonstrate benefits to patients who begin treatment early and avoid treatment interruptions
Reuters Report from CROI:
Early HIV Therapy Yields Better Outcomes and Fewer Toxicities
DENVER (Reuters Health) Feb 08 - HIV-infected patients may need to start highly active antiretroviral therapy (HAART) earlier than current US treatment guidelines suggest.
Treatment guidelines currently recommend delaying HAART until patients reach CD4 cell counts lower than 250 cells per microliter to avoid treatment toxicities. However, the results of a large study reported at the 13th annual Conference on Retroviruses and Opportunistic Infections found that patients who start treatment earlier and do not interrupt treatment actually have fewer toxicities and better outcomes.
"The intuitive thinking is the longer you are on a drug, the more the exposure to the drug, the greater the risk of developing toxicity will be," lead investigator Dr. Kenneth Lichtenstein of the University of Colorado Health Center, Denver told Reuters Health.
"What we have found is that it's the exact opposite. If you're going to develop toxicity, you develop it early, and it usually is associated with more advanced disease. If you don't develop it in the first year, your risk of developing it in the second year is lower and the risk continues to go down over time."
Dr. Lichtenstein and colleagues at the Centers for Disease Control and Prevention in Atlanta and Cerner Corp in Vienna, Virginia, evaluated 2222 patients in the HIV Outpatient study cohort who were seen at least twice between 1996 and 2005.
The subjects were stratified by pretreatment CD4 cell count, time of HAART initiation, and the development of three major toxicities.
Overall, 113 patients developed renal insufficiency, 301 developed peripheral neuropathy and 176 had lipoatrophy.
Compared with patients who started HAART when CD4 counts were 200 cells per microliter or lower, patients with CD4 counts higher than 350 cells per microliter when they began treatment were at least 60% less likely to develop renal insufficiency. They were also at least 60% less likely to develop lipoatrophy and 30% less likely to develop peripheral neuropathy.
Adherence to treatment also affected outcomes. CD4 counts were higher over an 8-year period in patients who took HAART 95% of the time or more than patients who took their drugs less frequently -- regardless of pre-HAART CD4 level. Similarly, compared with less compliant patients, more patients who took HAART at least 95% of the time had viral loads below 50 copies/mL (57% vs 36%; p < 0.01).
Patients who stayed on treatment 95% of the time or more had a lower incidence of HIV-related mortality and morbidity and appeared to have a lower risk of developing some toxicities, compared with patients who were less compliant, regardless of when HAART began. Less consistent use of therapy was also associated with an increased risk of renal insufficiency and peripheral neuropathy, but the risk of lipoatrophy was decreased.
Dr. Lichtenstein believes the committee that advises Health and Human Services on HIV treatment guidelines should consider the results of this study, along with those of other studies with similar findings.
"I wouldn't change the guidelines based on this study alone," he said. He also stressed that this was an epidemiological study, not a randomized, controlled trial. However, "a lot of the same kinds of things were seen in large structured treatment interruption studies that were randomized, controlled trials."
As a clinician, Dr. Lichtenstein starts his patients on HAART early. "I have very few patients hospitalized and very few complications. Although this is anecdotal," he commented, "it's consistent with the data."
The Program abstract reported this:
Background: Concern about HAART-related toxicities have led to guidelines that advocate delayed initiation of therapy. Using the HOPS cohort, we analyzed the response to HAART and the incidence of selected toxicities by pre-HAART CD4 cell count (pHCD4) and by time on HAART once initiated.
Methods: We analyzed a prospective, dynamic cohort of >7800 patients followed since 1993. We stratified patients by pHCD4 (0 to 49, 50 to 199, 200 to 349, 350 to 499, and >500 cells/mm3) and by time on HAART after initiation (took ≥95% of the time vs <95% of the time) and before development of toxicities. We used c2 and logistic regression analyses to assess risks for developing three toxicities: renal insufficiency, peripheral neuropathy, and lipoatrophy in the HAART era.
Results:
Of 2304 patients seen in HOPS clinics at least twice from 1996 to June 30, 2005, there were 124 with renal insufficiency, 278 with peripheral neuropathy, and 168 with lipoatrophy.
Approximately 31% of patients received HAART <95% time during follow-up. For each pHCD4R, after 8 years on HAART, CD4 count responses were higher (p <0.001) and the percentage of patients achieving viral loads <50 copies/mL greater (p <0.002) for the ≥95% group vs the <95% group. Incidence of death and of AIDS-defining illnesses decreased with higher pHCD4 (p <0.01) and at each pHCD4 was lower in the ≥95% group vs <95%.
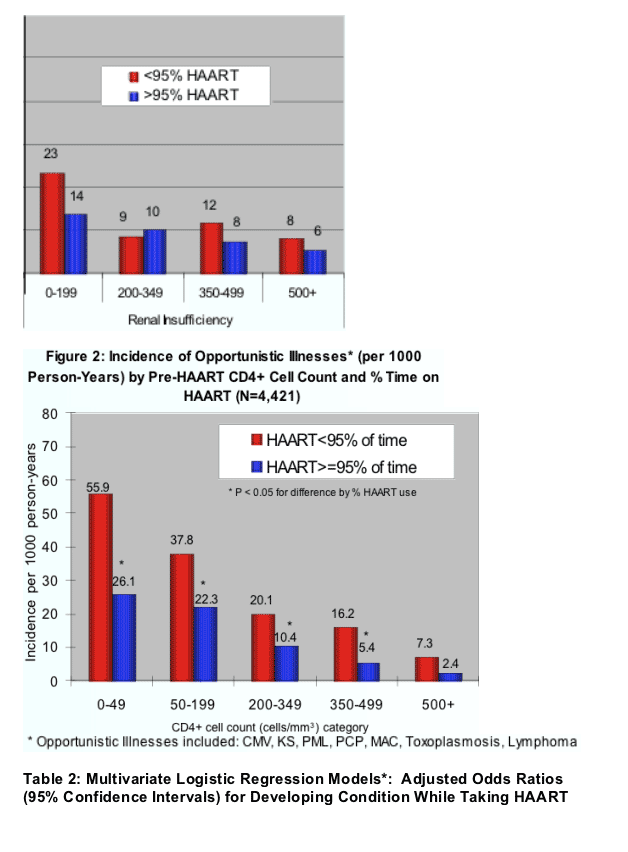
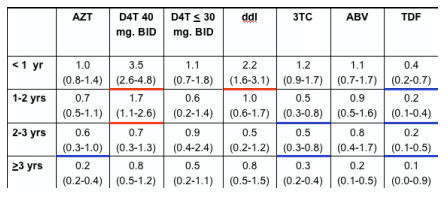
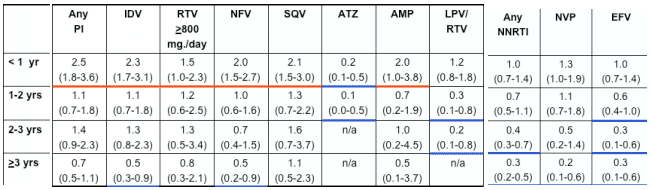
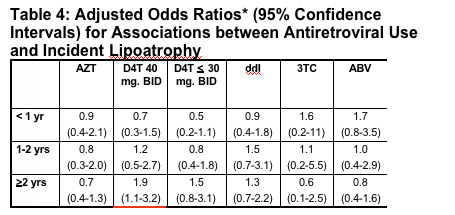
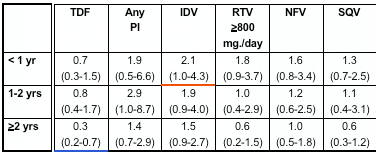
In multivariate analyses of demographic and clinical factors associated with incident toxicities, persons with higher pre-HAART CD4+ cell counts were consistently less likely to develop these toxicities (Table 2, see table below). In addition, persons who took HAART <95% of time were more likely to develop renal insufficiency and distal symmetrical polyneuropathy than persons who took HAART ≥95% of time; however, the reverse association was seen for lipoatrophy.
Univariate analyses found significantly decreased risk for renal insufficiency, peripheral neuropathy, and lipoatrophy among patients initiating HAART at higher pHCD4 and who remained on HAART ≥95% of the time.
In multivariate analyses, independent (p <0.05) risk factors for each outcome were as follows (adjusted odds ratio shown in parentheses):
for renal insufficiency, pHCD4 200 to 349 (0.6), 350 to -499 (0.4), >500 (0.3); <95% time on HAART (1.9); hypertension (2.1); African American race (1.6); body mass index <21 (1.9); ART-naive (2.9);
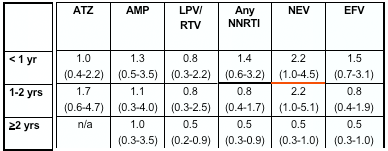
for peripheral neuropathy, pHCD4 200 to 349 (0.7), 350 to 499 (0.6), >500 (0.7); <95% time on HAART (1.5); age >40 years (1.3); stavudine 40 mg twice daily (1.8); any protease inhibitor (PI) (1.3); and for lipoatrophy, pHCD4 200 to 349 (0.5), pHCD4 350+ (0.4) [poster reports: 0.4 200-349; 0.3 350-499; 0.5 >500], age >40 years (1.8), white race (2.7), stavudine 40 mg twice daily (1.8), didanosine (1.6), tenofovir (0.6), any PI (2.1).
Conclusions: HOPS patients with higher pre-HAART CD4+ cell counts were less likely to develop renal insufficiency, peripheral neuropathy, and lipoatrophy in the HAART era, suggesting the benefit of earlier HAART treatment. Our findings also raise the possibility that use of HAART without interruptions may reduce the incidence of these complications.
The Poster Reported This Information:
See interesting tables & graphs below following Conclusion, Results & Methods.
CONCLUSIONS
- Incidence rates of mortality and opportunistic illnesses are reduced, and CD4+ cell count and viral load responses are improved, in HOPS patients started on HAART at higher pre-HAART CD4+ cell counts.
- Patients are less likely to develop renal insufficiency, distal symmetrical peripheral neuropathy and lipoatrophy when they start HAART at higher CD4+ cell counts.
- Consistent use of HAART (i.e. without interruptions) may improve outcomes and reduce the incidence of complications.
RESULTS:
- Incidence of mortality and opportunistic illnesses was lower among patients with higher pre-HAART CD4+ cell count. Within each pre-HAART CD4+ cell count category, these rates were also lower among patients who took HAART consistently (>95% of time) compared with those who took HAART less consistently (<95% of time) (Figure 1 and 2).
- For each pre-HAART CD4+ cell count category, CD4+ cell count responses over an 8-year period were greater among patients who took HAART >95% of time compared with those who took HAART <95% of time (Figure 3). Likewise, among 513 patients who had paired viral load data, 36% of 193 patients who took HAART <95% of time vs. 57% patients who took HAART >95% of time achieved viral load <50 copies/ml at 8 years of HAART use (p<0.01).
- Of 2,222 patients seen in HOPS clinics at least twice from 1996 to June 30, 2005, 113 developed renal insufficiency, 301 developed distal symmetrical polyneuropathy, and 176 developed lipoatrophy (Table 1). The incidence of renal insufficiency and distal symmetrical polyneuropathy was reduced among patients initiating HAART at higher pre-HAART CD4+ cell count and among those who took HAART >95% of the time compared with those who took HAART <95% of time (Figure 4).
- In multivariate analyses of demographic and clinical factors associated with incident toxicities, persons with higher pre-HAART CD4+ cell counts were consistently less likely to develop these toxicities (Table 2). In addition, persons who took HAART <95% of time were more likely to develop renal insufficiency and distal symmetrical polyneuropathy than persons who took HAART ≥95% of time; however, the reverse association was seen for lipoatrophy.
- Use of select antiretrovirals influenced the risk of developing distal symmetrical polyneuropathy and lipoatrophy, with greater risk of toxicities during the first year or two of use for many agents (Figure 3 and 4). No associations were seen between the use of any antiretrovirals and incident renal insufficiency (data not shown).
Limitations
- Only a minority of patients had paired pre-HAART and 8-year CD4+ cell count and HIV viral load data, thus these long-term responses on HAART may not reflect responses for all HOPS patients.
Background
Concerns about HAART-related toxicities have led to guidelines that advocate delayed initiation of therapy. Using the HOPS cohort, we analyzed the response to HAART and the incidence of selected toxicities by pre-HAART CD4+ cell count and by time on HAART once initiated.
Methods
We analyzed a prospective, dynamic cohort of >8000 patients followed since 1993. We limited analyses to patients that had been taking a HAART regimen for a minimum of 90 days. We analyzed the following while patients were taking HAART :
a) Incidence of mortality and opportunistic illnesses
b) Changes in CD4+ cell count and HIV viral load among patients with paired data before starting HAART and after 8 years of follow-up
c) Incidence of renal insufficiency and distal symmetrical polyneuropathy
d) Associations between demographic and clinical characteristics and development of incident renal insufficiency, distal symmetrical polyneuropathy, and lipoatrophy in the HAART era.
- We stratified patients by pre-HAART CD4+ cell count range (0-49, 50-199, 200-349, 350-499, and >500 cells/mm3) and by time on HAART after initiation (took >95% of the time vs. ≦95% of the time) before patients developed these outcomes. We used chi-square and multiple logistic regression analyses to evaluate associations. Changes in CD4+ cell counts were assessed by Kruskal-Wallis test of medians.
- Renal Insufficiency: Patients were considered to have renal insufficiency if they had either a diagnosis of renal failure, end stage renal disease, HIV-related nephropathy, glomerulonephritis, or a creatinine level test result > 2.0 with creatinine clearance < 70 mL/min.
Distal Symmetrical Polyneuropathy: We identified HOPS patients who had a clinical diagnosis of distal symmetrical polyneuropathy, that included any of the following medical chart entries: "Peripheral Neuropathy: bilateral and distal", "Peripheral Neuropathy: Due to Treatment", "Peripheral Neuropathy: Due to HIV", "Peripheral Neuropathy: Other/unknown", "Pain, Tingling, or Numbness in hands and/or feet". Patients with radiculopathy, proximal, or asymmetric symptoms were treated in the analysis as not having distal symmetrical polyneuropathy.
Lipoatrophy : Three focused HOPS surveys were conducted to evaluate for physical evidence of fat maldistribution (lipodystrophy) using a standardized survey tool: Survey I (October -December 1998, N=1077); Survey II (July - September 2000, N=1244); and Survey III (September 2004 -September 2005, N=754) which was limited to patients who completed either Survey I or Survey II, or both. Of these patients, 361 patients had pre-HAART CD4+ and complete recorded antiretroviral history. Three body areas were evaluated for lipoatrophy in all surveys: superficial fat loss (thinning) of the extremities, thinning of the hips/buttocks, and sunken cheeks. Each sign was graded: "none", "subtle", "moderate", or "severe". For this analysis, patients were categorized as having moderate to severe lipoatrophy if they had either one severe sign or two more signs with at least one moderate or severe sign. Patients who developed moderate or severe lipoatrophy by any of the three survey date windows were compared with patients who had no signs or only mild physical signs of lipoatrophy up to and including the third survey date window.

|
|
| |
| |
|
 |
 |
|
|