 |
 |
 |
| |
Tipranavir RESIST Analysis
|
| |
| |
Reported by Jules Levin
13th CROI, Feb 2006, Denver
The results of this analyis were reported by Katlama et al in poster 520 at CROI.
"Tipranavir (TPV/r) achieves twice the rate of treatment responses and prolongs durability of response versus comparator PI (CPI/r) in ARV-experienced patients, independent of baseline CD4 cell count or VL. Week 48 RESIST 1 and 2 combined analyses"
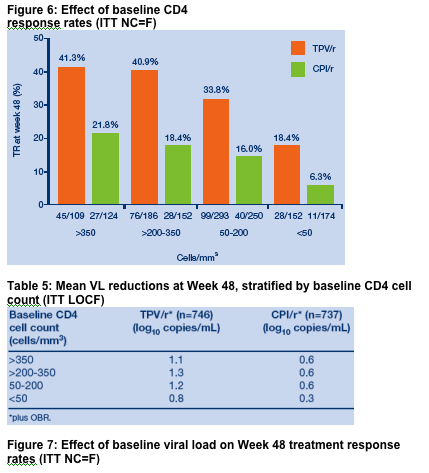
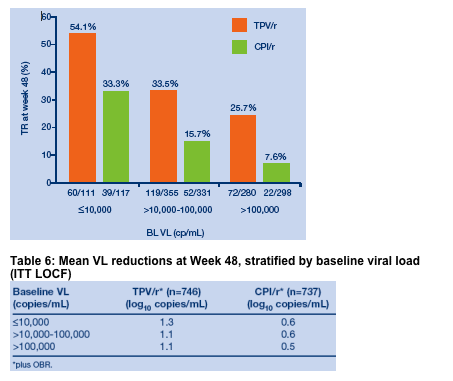
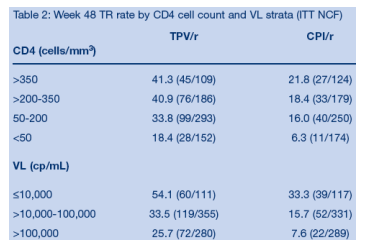
1400+ study patients were disease advanced and with extensive ART resistance: 38% >100,000 cp/ml; mean CD4 count: 195; 22% <50 cp/ml; 56% with history of AIDS defining illnesses; median no. of prior ARVs: 12, 4 (1-7) PIs, 1 (0-3) NNRTIs; prior ENF, T-20/Fuzeon use: 10%; no. of primary PI mutations: 3. This analysis reports several findings: a Kaplan-Meier curve estimate of Time To treatment Failure in the RESIST Studies is displayed below (HR at week 48=0.63 [p<0.0001]), displaying the durability of response out to week 72. Baseline viral load & CD4 count was associated with response to TPV; if patient VL was <10,000, 54% achieved a treatment response (>1 log VL reduction) to TPV/r compared to 33% for patients randomized to CPI/r arm. For patients with baseline VL 10,000-100,000, the treatment response was 33% for TPV/r vs 16% for CPI/r. For patients with >100,000 copies/ml at baseline, the treatment response was 26% for TPV/r vs 7.6% for CPI/r arm. Patients with baseline CD4 count of >350 had 41% treatment response after 48 weeks with TPV/r vs 22% for CPI/r; for patients with 200-350 CD4s the treatment response was 41% for TPV/r vs 18% for CPI/r; for patients with baseline CD4 of 50-200 treatment response was 33% for TPV/r vs 16% for CPI/r; and for patients with <50 CD4s the treatment response was 18.4% for TPV/r vs 6.3% for CPI/r.
C Katlama1, S Walmsley2, C. Hicks3, P. Cahn4, D Neubacher5, J. Villacian6 for the RESIST 2 and RESIST 1 Study Teams
1Hopital Pitie Salpetriere, Paris, France; 2Toronto General Hospital, University of Toronto, Canada; 3Duke University, Durham, NC, USA; 4Fundación Huésped, Buenos Aires, Argentina; 5Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA;
6Boehringer Ingelheim International GmbH, Germany D-55216
Introduction
Tipranavir (TPV, Aptivus) is a recently approved novel protease inhibitor (PI)
with potent activity against multiple PI-resistant HIV-1. TPV/r is effective and
well tolerated in PI experienced patients [1-3].
RESIST 1 and 2 are randomized, ongoing, open label, comparative Phase III
studies of TPV/r (500/200 mg BID) [2,3]. RESIST 1 is taking place in N America
and Australia; RESIST 2 is being conducted in Europe and Latin America.
The RESIST studies enrolled triple antiretroviral (ARV) class experienced
patients, who were followed for at least 48 weeks. Patients were randomized to
receive an optimized background regimen (OBR) plus TPV/r or a standard of
care, ritonavir boosted, comparator PI (CPI/r). Since the study designs of
RESIST 1 and 2 were similar, the data from both clinical trials were analyzed in
a combined analysis.
We present Week 48 results of the combined analysis of the RESIST data.
At Week 48, the total exposure to study medication was 737.9 patient-years in
the TPV/r arm and 459.2 patient-years in the CPI/r arm.
Durability of treatment responses to TPV/r
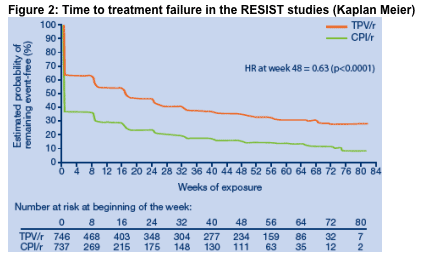
The risk for treatment failure through week 48 was lower in the TPV/r arm
compared to CPI/r (HR 0.63, p <0.0001) (Figure 2). The median TTF was 113
(Interquartile Range [IQR] 0->494) days in the TPV/r arm vs. 0 (IQR 0-119) days
in the CPI/r. These data demonstrate that more patients taking TPV/r plus an
OBR achieved a durable response than those taking a CPI/r plus OBR.
Effect of baseline viral load on ability to achieve a treatment
response at Week 48
The proportion of patients at Week 48 who experienced a TR was considerably
higher in the TPV/r arm compared to the control arm, regardless of the baseline
VL (Figure 7). Patients who initiated TPV/r therapy with lower baseline VLs
were more likely to respond than those with higher baseline VLs. More than
50% of patients who started TPV/r with a baseline VL of <10,000 copies/mL
experienced a TR.
Baseline VL was associated with the TR rate, even after controlling for PI
assignment (TPV or CPI) and ENF use.
CD4 cell responses
Patients in the TPV/r group experienced a greater increase in CD4 cell counts
compared with those taking a CPI/r (ITT Last Observation Carried Forward
[LOCF] analysis) (Table 7). These findings are important as changes of this
magnitude in virologic and immunologic markers have been associated with
a decreased incidence of AIDS-defining events [4].

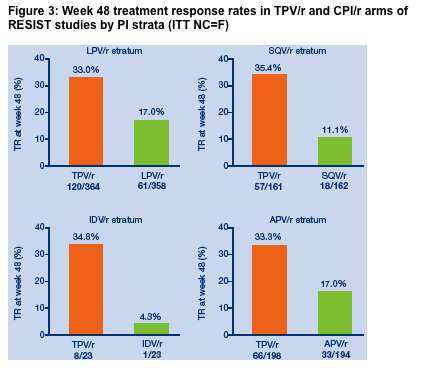
Twice as many TPV/r patients achieved a treatment response at
Week 48 as control patients in all PI strata
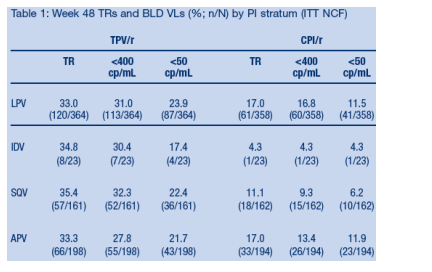
Week 48 TRs were superior in the TPV/r arm compared to the control arm,
regardless of the PI/r taken by the control patients (Figure 3). In all PI strata,
TPV/r out-performed the CPI/r by approximately two fold or more.
Relatively few patients were assigned to the indinavir stratum and so the power
of this comparison is limited.
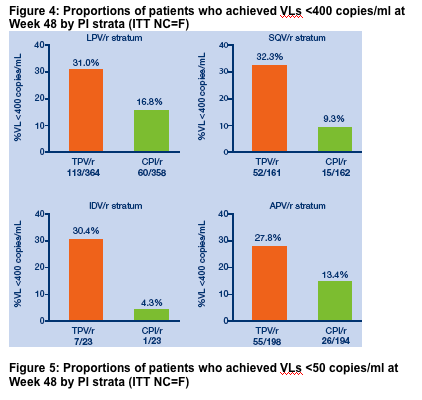
Figure 3: Week 48 treatment response rates in TPV/r and CPI/r arms of
RESIST studies by PI strata (ITT NC=F)
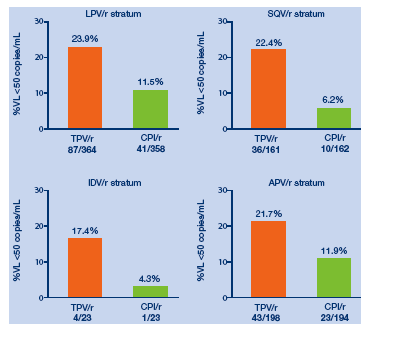
The proportions of patients in each PI strata who achieved viral loads <400 or
<50 copies/mL are shown in Figures 4 and 5. In all PI strata, the proportion of
patients with undetectable VLs was approximately two fold or higher in the
TPV/r arm than in the CPI/r arm.
Abstract
Background: TPV/r, a potent agent against multi-PI-resistant HIV, is now
approved in several countries. We present Week 48 treatment responses (TR)
to TPV/r or CPI/r regimens in ARV experienced, RESIST 1 and 2 patients,
stratified by PI, baseline (BL) CD4 cell counts and BL VLs.
Methods: RESIST 1 and 2 are randomized, ongoing, open label Phase III
studies of TPV/r (500/200 mg BID) or CPI/r, plus OBR. Patients had 33-class
ARV experience, 32 previous PI-based regimens, >/= 1 primary PI mutation and
<3 mutations among 33, 82, 84 and 90. CPI/r and OBRs were selected prerandomization.
TR rates (confirmed >/= 1 log10 copies/mL VL reduction without
treatment change) at Week 48 were determined for PI, BL CD4 count and BL
VL strata, in a combined analysis.
Results: 1483 patients were randomized: 746 to TPV/r; 737 to CPI/r.
Baseline mean CD4 cell counts were 196/195 cells/mm3 and mean VLs were
4.73/4.73 log10 copies/mL in TPV/r and CPI/r arms respectively.
Median prior ARVs were 12. Pre-selected PIs were lopinavir (LPV; 722), saquinavir (SQV; 323), amprenavir (APV; 392) and indinavir (IDV; 46). 20.5% of patients took enfuvirtide.
Risk of treatment failure was 39% lower in TPV/r vs. CPI/r patients (p <0.0001).
Median TTF was 113 days in TPV/r (IQR 0->494) vs. 0 in CPI/r (IQR 0-119):
TPV/r responses were more durable.
Conclusions: Week 48 TR rates were superior in TPV/r patients vs. CPI/r,
regardless of PI/r, baseline VL or CD4 cell count. Initiating TPV/r in ARV
experienced patients with lower BL VLs or higher BL CD4 cell counts improved
virological responses.
Study design and patients
RESIST 1 and 2 enrolled male and female patients with HIV infection who
fulfilled the following entry criteria:
- 318 years old
- 33 consecutive months’ experience with 3 classes of ARV drugs (NRTIs,
NNRTIs, PIs)
- 32 PI-based regimens for 33 months; one of which was the current
treatment regimen
- Any CD4 cell count
- Viral load (VL) >/= 1,000 copies/mL
- Viral isolate carrying 31 primary protease mutation at 30N, 46I/L, 48V, 50V,
82A/F/L/T, 84V, 90M
- Viral isolate carrying <3 mutations at codons 33, 82, 84, 90
The investigators selected the CPI/r (lopinavir/r, indinavir/r, saquinavir/r or
amprenavir/r) and the OBR prior to randomization (Figure 1). The use of
enfuvirtide (ENF) was allowed; patients were stratified by ENF usage, as well as
by the CPI/r (the PI stratum).
CPI/r patients who failed virologically after 8 weeks were able to switch to
a long-term TPV/r safety study.
Patients received 500/200 mg TPV/r or standard doses of the CPI/r plus
approved doses of the components of the OBR.
After 8 weeks, patients who failed virologically in the CPI/r arm were able to
receive TPV/r prior to licensing via a long term safety study, provided that there
was documented evidence that they had been adherent to their study
medication.
Treatment response (TR) rates (confirmed VL reduction 31 log10 copies/mL at
Week 48 without viral rebound; prior treatment change; study discontinuation,
including loss to follow-up; or death) and Time to Treatment Failure (TTF) were
compared for patients taking TPV/r and those taking CPI/r.
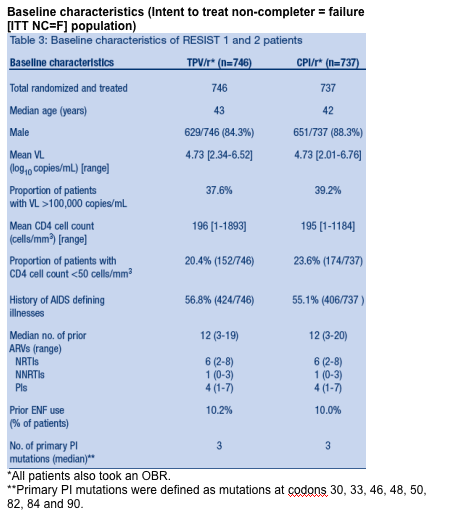
Results
1483 patients received TPV/r or CPI/r plus OBR for 348 weeks. Baseline
characteristics were similar in both studies and both treatment arms (Table 3).
|
|
| |
| |
|
 |
 |
|
|