 |
 |
 |
| |
Safety and Efficacy of Adefovir Dipivoxil in Patients with Lamivudine-Resistant Chronic HBV Undergoing Liver Transplantation
|
| |
| |
Reported by Jules Levin
DDW, May 21-25, Los Angeles, 2006
Eugene Schiff (University of Miami, FL, USA) reported these data at both EASL & DDW.
Author Conclusions
1. Adefovir dipivoxil appears to be effective prophylaxis for graft re-infection with or without HBIg.
2. Adefovir dipivoxil was safe and generally well-tolerated in liver transplantation patients.
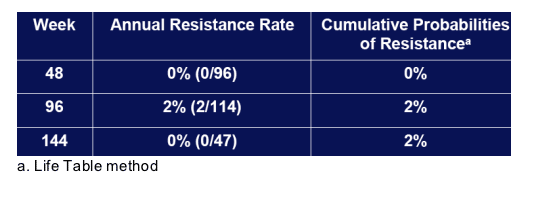
3. Cumulative probabilities of resistance were 0%, 2% and 2% at weeks 48, 96 and 144.
HBV Therapy is Warranted for Patients with Chronic HBVC Undergoing Liver Transplantation
Re-infection can lead to progressive liver disease in the graft.
Re-infection of the graft following liver transplantation for chronic hepatitis B (CHB) frequently occurs in the absence of antiviral therapy.
The risk of re-infection is decreased with lower viral titers pre-transplantation.
Hepatitis B Immune Globulin (HBIg)
-Effective prophylaxis for graft re-infection
-Costly, parenteraladministration, escape mutations can arise
Lamivudine
-Effective in lowering viral titers pre-transplantation and post-transplantation
-Effective prophylaxis for graft re-infection
-Frequent development of resistance
Adefovir Dipivoxil (ADV)
-Effective in lowering viral titers pre-transplantation and post-transplantation
Interferon alpha
-Risk of serious adverse effects in patients with decompensatedcirrhosis
-Risk of graft rejection post-transplantation
Entecavir
-Safety and efficacy in liver transplant recipients unknown
Background
Study 435 was an international, compassionate use study of the safety and efficacy of ADV for the treatment of wait-listed (n=226) and post-transplantation patients (n=241) with lamivudine-resistant chronic hepatitis B.
Study objective
Investigate the prevention of HBV graft re-infection using ADV.
Methods
Patients with lamivudine-resistant CHB were included if they underwent transplantation during Study 435.
Patients were being treated with adefovir dipivoxil (ADV) with or without ongoing lamivudine prior to transplantation.
Patients were treated with or without HBIg following transplantation.
Serum HBV DNA was centrally assessed using the Roche Amplicorassay with a lower limit of quantification (LLQ) of 1000 copies/mL.
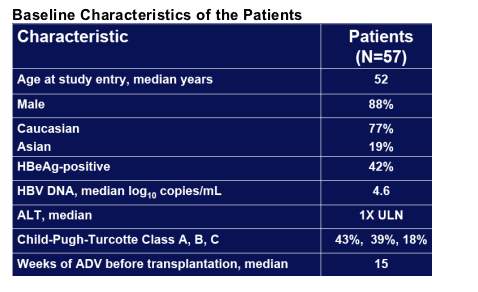
Post-Transplantation
Treated and followed on ADV for median 36 weeks
HBIg given to 34 (60%) patients; 23 did not receive HBIg
No statistically significant (p<0.05) differences between patients receiving or not receiving HBIg in:
- Demographics
- Last serum HBV DNA pre-transplantation
- No HBIg-mean (±SD) HBV DNA 4.78 (±1.67)log10copies/mL
- HBIg-mean (±SD) HBV DNA 4.80 (±1.72)log10copies/mL
- Last ALT pre-transplantation
- HBeAg status
- CPT class distribution
- Weeks of ADV
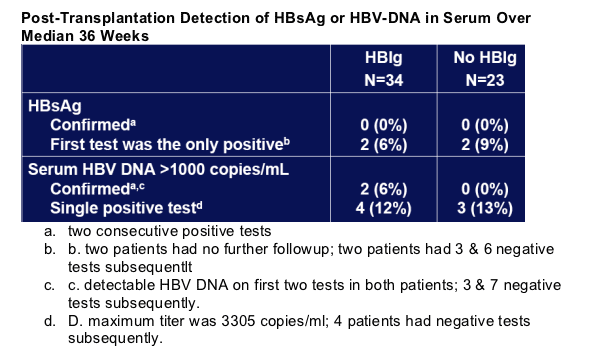
Graft Re-infection
Re-infection was defined a priori as confirmed detection of HBsAg or HBV DNA in serum.
No patient had confirmed detection of both HBsAg and serum HBV DNA.
Safety
Four patients (7%) discontinued ADV due to an adverse event; none was judged related to ADV.
In these patients also on nephrotoxic immunosuppressant drugs, the highest measurement of serum creatinine post-transplantation was:
-Normal or grade 1 in 84% patients
-Grade 2 in 7% patients
-Grade 3 in 7% patients
-Grade 4 in 2% patients (grade 4 at baseline)
Resistance
None of the patients who underwent on-study liver transplantation developed resistance over a mean of 67 weeks of ADV.
Long-term rates estimated from annual surveillance for resistance in pre-and post-transplantation patients in Study 435 (n=129):
|
| |
|
 |
 |
|
|