 |
 |
 |
| |
The Effect of Liver Fibrosis and Cirrhosis on SVR in 4913 Patients With Hepatitis C: Results From The WIN-R Trial
|
| |
| |
"....Cirrhosis remains an important predictor for SVR in HCV patients...It is important to use weight-based dosing of ribavirin to increase SVR in patients with advanced fibrosis, especially for cirrhosis.....Cirrhosis (F4) is the major histological determinant of SVR according to F stage..."
Reported by Jules Levin
DDW, May 2006, Los Angeles
Authors: N. Afdhal, I. Jacobson, R. Brown, B. Freilich,
J. Santoro, L. Griffel, C. Brass, and the WIN-R Study Group
Background from authors
Results from multiple studies suggest that advanced fibrosis is a negative predictor of SVR in treatment-naive patients who receive PEG-IFN alfa plus RBV
Most studies are small and do not contain a significant proportion of patients with cirrhosis. Patients with F3 fibrosis and cirrhosis (F4) are often analyzed together rather than separately
WIN-R Trial background
- PEG IFN alfa-2b 1.5 _g/kg/week + RBV 800 mg/day was superior to 2 other regimens using RBV 1000 to 1200 mg/day in a pivotal trial1
- Logistic regression analysis showed a linear relationship between RBV dose in mg/kg and SVR
- Secondary analysis showed superiority of weight-based dosing of RBV
- A large prospective trial comparing RBV doses was designed to address fixed-dose RBV 800 mg versus weight-based RBV dosing (Weight-Based Interferon and Ribavirin Trial)
- Win-R: Investigator-initiated access trial involving academic and community sites in the United States
- 25 regional investigators, 225 sites
- Nearly 5000 patients evaluated; 500 with cirrhosis*, *Out of 4913 patients in the primary safety analysis.
- Central randomization stratified by genotype and fibrosis
- Participant criteria
- Compensated liver disease, biopsy results consistent with chronic hepatitis C
- At least 1 elevated alanine transferase level in 6 months before entry
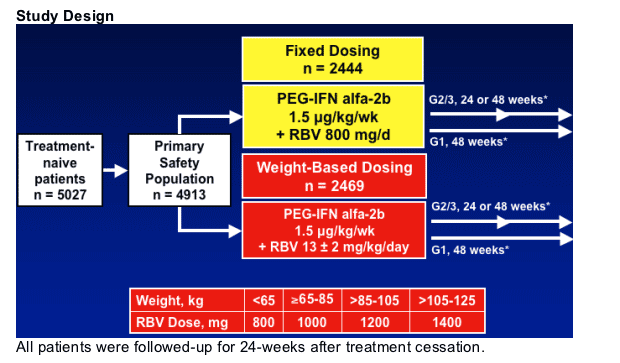
Study Populations and Analyses
- Intent-to-treat population (n = 4913)
- All subjects who received at least one dose of study drug
- Used for safety analysis
- Primary efficacy population (n = 4223)
- All subjects who received at least one dose of study drug and weighed >/=65 kg
- Used for primary efficacy analysis - SVR
Hypothesis:
Fibrosis Is an Independent Predictor
of SVR
Aim:
To Look at the Effect
of Individual and Combined
Fibrosis Stages on SVR Rates
Methods
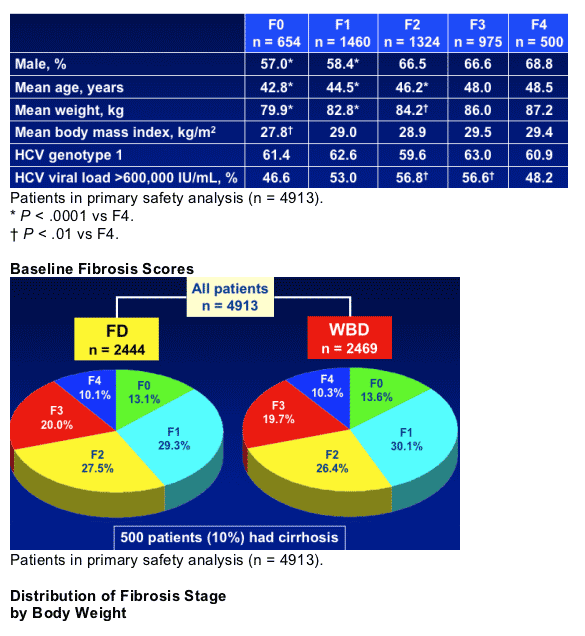
- Classification of fibrosis stage was determined by the principal investigator and local pathologist using METAVIR scoring*
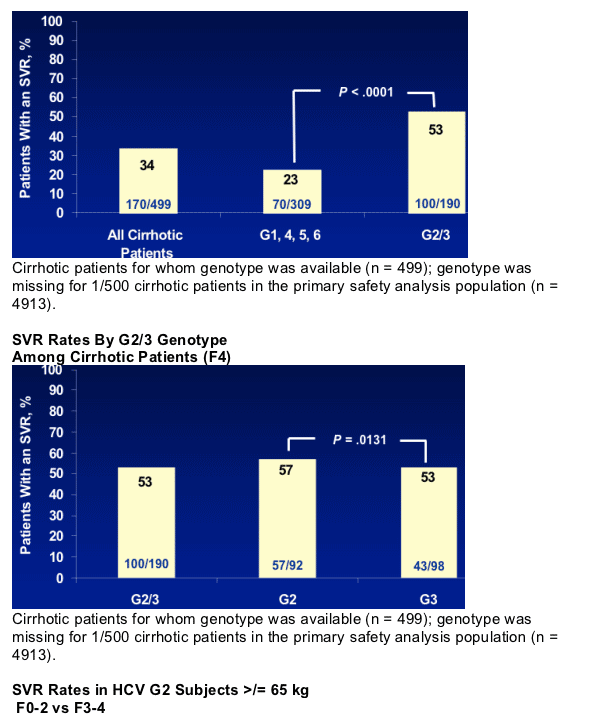
*F0 = none; F1 = portal fibrosis; F2 = few septa; F3 = many septa; F4 = cirrhosis.
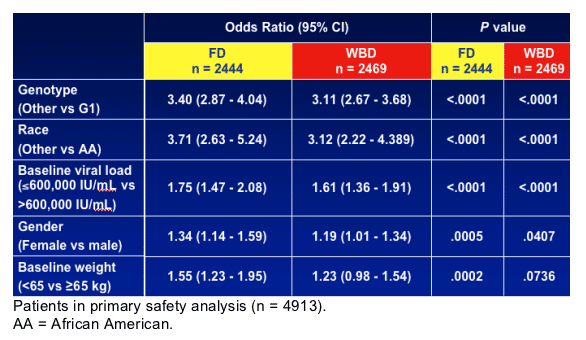
- Univariate and multivariate logistic regression analyses were conducted for fibrosis and SVR
- Analysis was based on patients (n = 4913) who received at least one dose of study drug
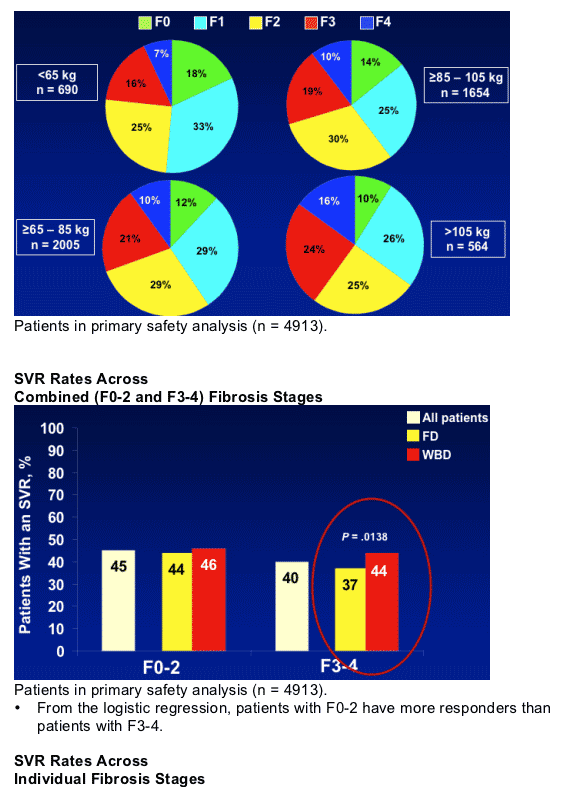
WIN-R Population: Demographics
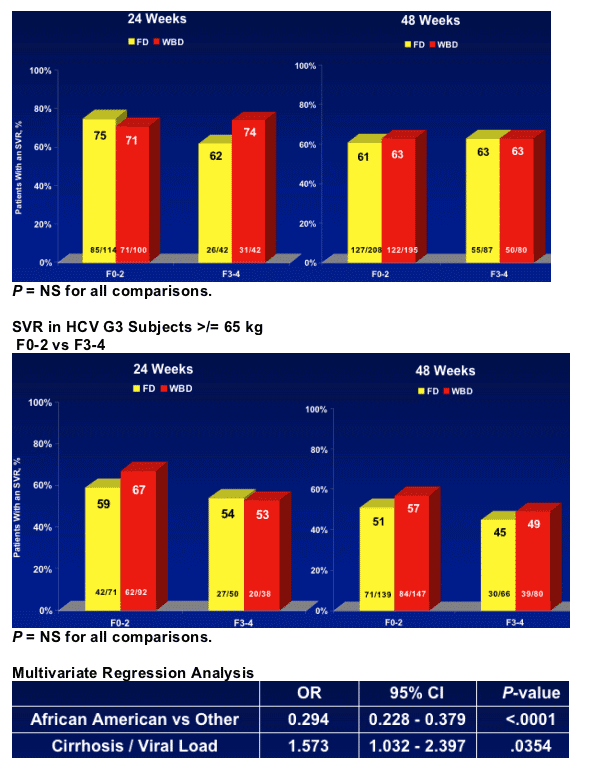
- A significant interaction was found between cirrhosis and viral load (P = .0354) where cirrhosis negatively impacted SVR in LVL patients
- African Americans were 0.3 times as likely to respond to treatment as non-African Americans (P < .0001)
Summary
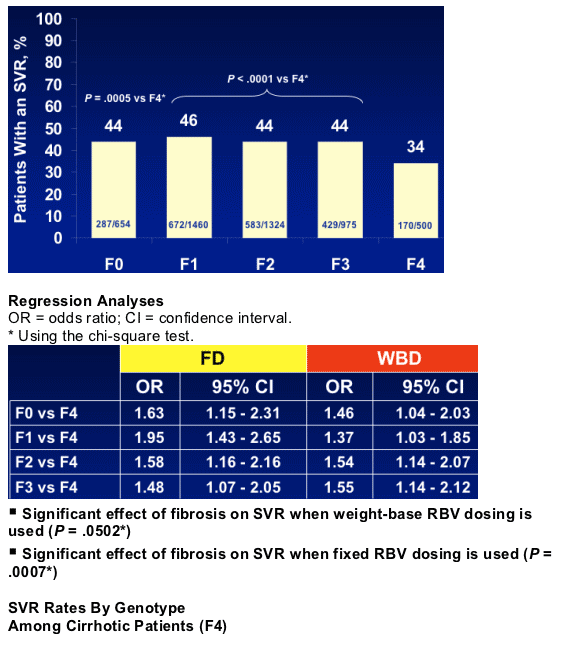
- It is important to use weight-based dosing of ribavirin to increase SVR in patients with advanced fibrosis, especially for cirrhosis
- Cirrhosis (F4) is the major histological determinant of SVR according to F stage
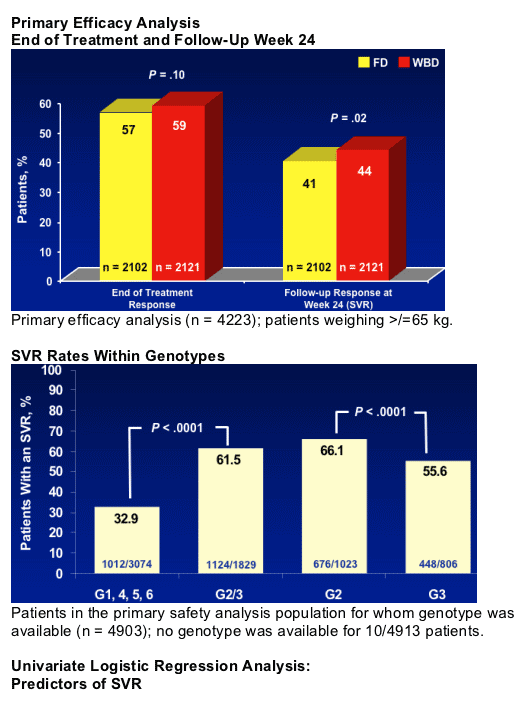
- Treatment for 24 or 48 weeks in genotype 2 and 3 has a similar SVR, independent of the presence of advanced fibrosis F3-F4
- Genotype 3 patients with cirrhosis have significantly reduced SVR compared to genotype 2
Conclusion
- Cirrhosis remains an important predictor for SVR in HCV patients
- Clinical trials need to continue to determine the % of patients with cirrhosis either by biopsy or non-invasive techniques
|
| |
|
 |
 |
|
|