 |
 |
 |
| |
High Barrier To Resistance Results In No Emergence Of Entecavir Resistance
In Nucleoside Naive Subjects During The First Two Years Of Therapy
|
| |
| |
Reported by Jules Levin
41st Annual Meeting of the European Association for the Study of the Liver (EASL) April 29, 2006, Vienna, Austria
These data were presented in poster 490 by: R.J. Colonno, R.E. Rose, C.J. Baldick, S.M. Levine, K. Klesczewski and D.J. Tenney.
Bristol-Myers Squibb Pharmaceutical Research Institute, Wallingford, CT,
All authors are employees of Bristol-Myers Squibb Company
UPDATED ABSTRACT
Background: Entecavir (ETV) is a highly effective inhibitor of hepatitis B virus (HBV) replication. Lamivudine-resistance (LVDr) substitutions (M204V/I ± L180M) reduce ETV susceptibility 8-fold, while virologic rebound due to ETV resistance (ETVr) requires preexisting LVDr substitutions plus additional changes at RT residues T184, S202 and/or M250 that can be selected by LVD.
Methods: Randomly selected patients (81%) were genotyped at baseline and Week 48. ETV-treated patients experiencing virologic rebounds (>/= 1 log increase from nadir by PCR) or failing to reduce their HBV DNA levels to <300 copies/ml by Week 96 in studies AI463-022, AI463-027 and AI463-901, were analyzed for emerging ETVr by genotypic analysis and phenotypic susceptibility assays.
Results: There was no evidence of resistance emergence by Week 96 of ETV therapy in any nucleoside naive patient lacking LVDr substitutions at study entry. Among 664 treated nucleoside-naive, HBeAg pos & HBeAg neg patients with PCR measurements, 91% achieved HBV DNA reductions to <300 copies/ml by Week 96. Sequencing of 81% of patients at study entry and Week 48 identified 71 RT residues with emerging changes. However, none reduced entecavir susceptibility or were present in >1.3% of patients. Genotypic analysis of all 18 virologic rebounds observed by Week 96 and 5 "suboptimal" responders (HBV DNA >105 copies/ml [100.000]) failed to show any evidence of emerging ETVr
substitutions, and population phenotypes at the time of rebound were essentially
unchanged from baseline. Viruses from any remaining patient who had not achieved reductions in HBV DNA levels to <300 copies/ml at the Week 96 time point (n=26) or at the time of study discontinuation (n=7) were also analyzed for resistance emergence. Interestingly, many of these Week 96 patients had HBV levels exceeding a billion copies/ml at the time of study entry. Thirteen of the twenty patients who continued on ETV therapy subsequently exhibited reductions in HBV DNA levels to <300 copies/ml in Year 3.
Summary: There was no evidence of resistance by Week 96 in ETV-treated nucleoside naive patients. The lack of ETVr is likely due to the rapid and sustained suppression of viral replication, high intracellular concentrations of ETV-triphosphate, and a requirement of multiple substitutions to achieve meaningful resistance level s. Such a high resistance barrier supports using ETV as a first-line treatment for HBV-infected patients.
Monitoring for ETV Resistance
- Genotyped all available samples from Study AI463-022 (n=340) and 66% of
randomly selected samples from Study AI463-027(n=215) patients on ETV at Study Entry and Wk 48
- Determined the resulting phenotype for changes at all 71 residues with
emerging substitutions identified on ETV therapy
- Genotyped & phenotyped samples from all patients experiencing suboptimal response (>105 copies/ml [100,000] at Week 96 or End of Treatment on ETV (n=5) and all virologic rebounds (=1 log increase from nadir by PCR) regardless of study arm
- Genotyped available samples from all remaining patients having detectable
HBV DNA (>300 copies/mL) at Week 96 (n=26) or End of Treatment (n=7)
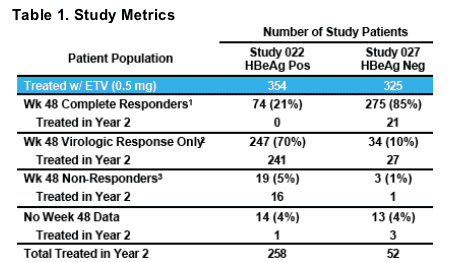
1HBV DNA <0.7 MEq/mL (bDNA) + loss of HBeAg (Study 022) or normalization of ALT (Study 027 )
2HBV DNA <0.7 MEq/mL (bDNA)
3HBV DNA >0.7 MEq/mL (bDNA)
- 94% of eligible patients classified as "Virologic Response Only" or "Non-
Response" continued therapy in Year 2 and were monitored for resistance
Median HBV DNA Levels of Nucleoside Naive, HBeAg Positive
and Negative Patients Treated with ETV
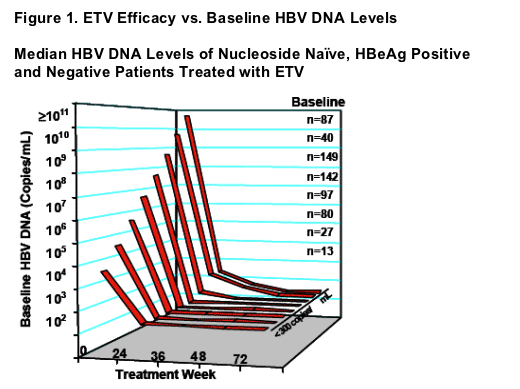
- ETV efficacy independent of baseline HBV DNA levels
- 348/369 (94%) patients with baseline HBV DNA levels <109 copies/mL (and a Week 48 PCR measurement) achieved reductions to <300 copies/mL
- 108/141 (77%) patients with baseline HBV DNA levels >109 copies/mL (and a Week 96 PCR measurement) achieved reductions to <300 copies/mL
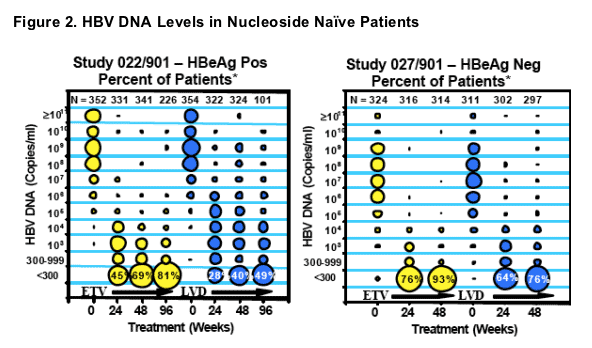
- More rapid and superior decrease in HBV DNA levels on ETV therapy relative to patients treated with LVD
- Continued decrease in HBV DNA levels during Year 2 of ETV treatment, compared to a more marginal decrease on continued LVD therapy
*The size of each circle corresponds to the percentage of patien ts at each time point having the log10 level of HBV DNA indicated, with each column of circles ad ding up to 100%.
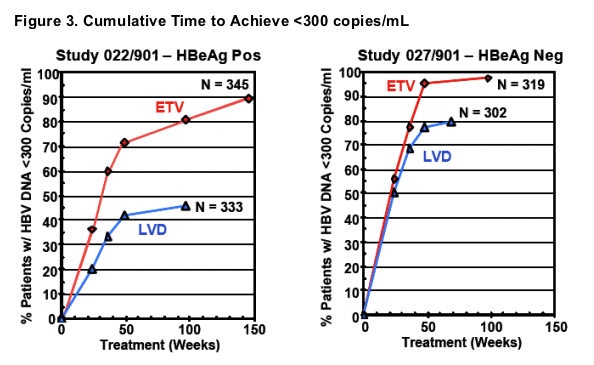
- Cumulative evaluation of 664 nucleoside naive, HBeAg positive & HBeAg negative ETV treated patients with PCR measurements on treatment showed that 91% achieved HBV DNA reductions to <300 copies/mL by Week 96
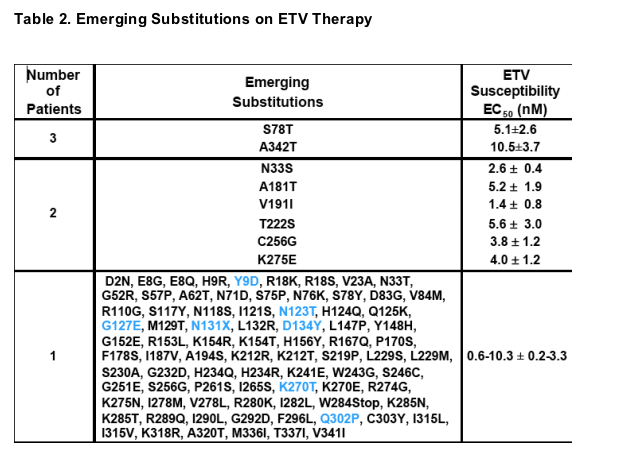
- Novel amino acid substitutions emerging on ETV therapy that were not observed in >99% of HBV in GenBank library of 250 WT HBV sequences
- Control WT HBV ETV EC50 levels were generally 4.3 ± 2.7 nM
- Substitutions located throughout the RT gene
- Changes only observed beyond Week 48 are shown in blue
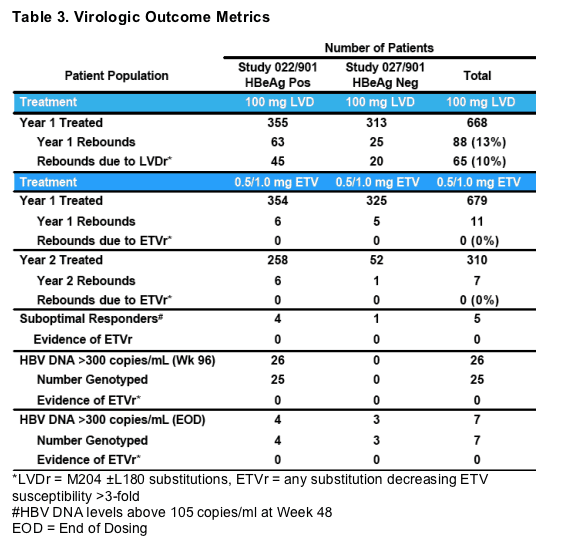
*LVDr = M204 ±L180 substitutions, ETVr = any substitution decreasing ETV susceptibility >3-fold
#HBV DNA levels above 105 copies/ml at Week 48
EOD = End of Dosing
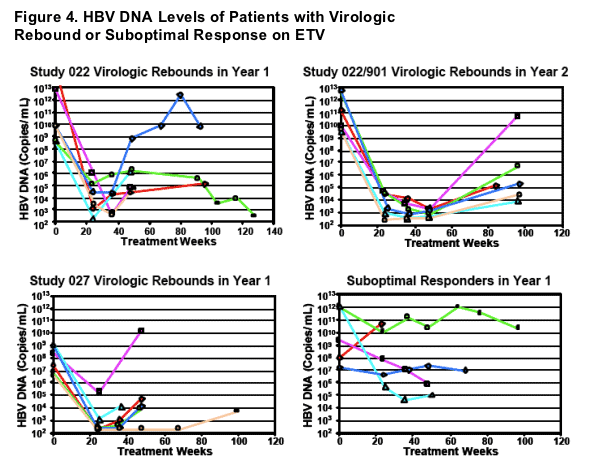
- Only 2 of 18 virologic rebound were confirmed by repeated assay
- Only 5 virologic rebounds exceeded 106 HBV DNA copies/mL
- Suboptimal Response = Patients not achieving HBV DNA reductions below 105 copies/mL (100,000) at Wk 48 or last on treatment
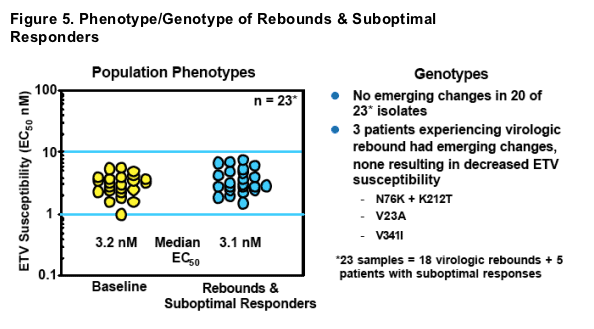
- No genotypic or phenotypic evidence of resistance emergence in RT
- Phenotypic assays on full viral genomes yielded similar results, suggesting virologic rebounds were not due to changes outside of the polymerase gene
- Observed rebounds most likely due to non-compliance
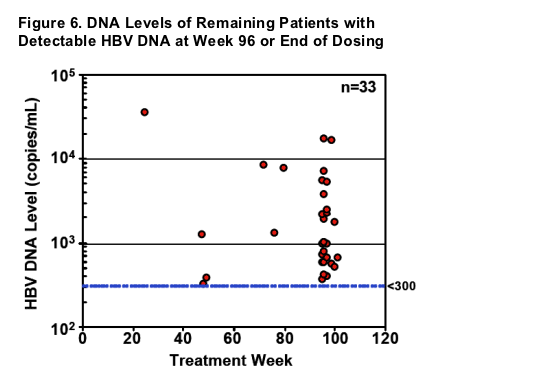
- Of the 33 patients with HBV DNA levels >300 copies/mL, all were less than 105 copies/mL and only 3 were above 104 copies/mL
- Of the 20 who continue into Year 3, 13 achieve <300 copies/mL
Summary and Conclusions
High genetic barrier to resistance is created in nucleoside naive patients treated with 0.5 mg ETV daily due to:
- Rapid and sustained suppression of viral replication-
- 91% of 664 ETV treated patients achieved reductions in their HBV DNA levels to <300 copies/mL by Week 96
- HBV DNA suppression continues to increase during the second year of therapy
- Requirement of 3 substitutions to achieve meaningful resistance levels-
- Virologic rebound due to ETV resistance only observed when LVD resistant viruses containing M204I/V ± L180M substitutions acquire additional substitutions at residues T184, S202 or M250
- None of the substitutions that emerge in nucleoside naive patients result in reduced susceptibility to ETV
- Virologic rebounds and suboptimal responses on ETV therapy not due to resistance emergence-
- None of the 18 virologic rebounds or 5 suboptimal responders were due to emergence of resistance substitutions or decreased ETV susceptibility
- Sequencing of 33 remaining patients with detectable HBV DNA (>300 copies/mL) at Week 96 or end of dosing failed to identify any ETV resistance substitutions
Combination of potent suppression of viral replication combined with lack of resistance at 2 years support the use of ETV as first line therapy
|
| |
|
 |
 |
|
|