 |
 |
 |
| |
Phase II Multi-Center, Dose-Escalating study of LB80380 (ANA380) in hepatitis B Patients with Lamivudine-Resistant YMDD Mutant HBV
|
| |
| |
Reported by Jules Levin
EASL April 2006, Vienna, Austria
C L Lai of Queen Mary's Hospital in Hong Kong, China, presented data from a Phase 2 dosing study of ANA380 at the 41st EASL conference in Vienna, Austria.
Summary: total of 65 lamivudine-refractory patients were enrolled and 62 completed 12 weeks with ANA380. 23 patients remain on the continuation treatment with adefovir. No clinically relevant adverse events observed during the treatment and the subsequent followup. 2.8, 3.2, 3.9, and 4.1 log of mean HBV-DNA reduction at 12 weeks were observed in 30 mg, 60 mg, 150 mg, and 240 mg dose groups of ANA380, respectively. The author concluded these doses for 12 weeks appear safe, and well-tolerated. Since 90, 150, and 240 mg doses yielded similar viral suppression development will focus on the lower end of this dose range.
ANA380 (LB80380) is an ester drug of phosphonate nucleotide analogue of guanosine monophosphate with potent activity against HBV. It's a prodrug of ANX331 (LB80331). LB80331 is further metabolized to LB80317 (ANX317), an analogue of guanosine monophosphate. LB80317 is further phosphorylated to tri-phosphate: inhibits viral replication following incorporation into viral DNA.
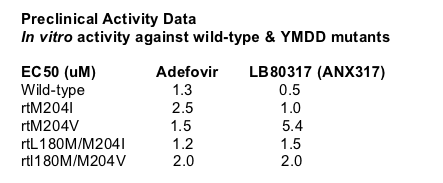
In a previous 4 week study three dose groups-60 mg, 120 mg, and 240 mg dosing --showed HBV DNA reduction of about -3.5 to -3.9 log reduction.
The study reported at EASL Vienna 2006 investigated the antiviral activity of ascending 12-week oral doses of ANA380 in chronic YMDD mutant HBV patients. Safety and tolerability of ascending multiple doses of ANA380 was also investigated in chronic YMDD mutant HBV patients. This is a phase II, multi-center, multi-national, open-label, sequential group, dose-escalating study.
Results of the clinical trial, which investigated the safety, tolerability and anti-viral activity of ANA 380 are based on an analysis of data in 62 patients in five cohorts. Cohorts received ANA 380 in escalating doses of 30 mg, 60 mg, 90 mg, 150 mg or 240 mg, once daily by oral administration for 12 weeks. Patients in each cohort had been previously treated with lamivudine, the current standard of care for HBV patients, and were documented to have genetically-encoded lamivudine resistance.
Dose escalation based on dose limiting toxicity during the first 4-week treatment. In patient groups 4 (150 mg) and 5 (240 mg), 660 mg and 990 mg of L-carnitine was supplemented along with ANA380 during the treatment period. It appeared from the fast moving slides that 380 was added to LAMivudine for 4 weeks followed by 8 weeks of 380 alone, followed in turn by adefovir for 12 weeks.
Patients were included if HBsAg+ for at least 6 months, HBeAg+ for at least 1 month; HBV DNA >106 (1 million) (Amplicor HBV Monitor assay); ALT1.5 to 10 x ULN; confirmed YMDD mutants (M204V/I with or without L180M); refractory to lamivudine therapy over 6 months. Followup was monthly till 24 weeks post treatment.
Patients were Asian. Mean age was 38-49 yrs. Mean HBV DNA was 7.9 to 8.3 log copies/ml. Median ALT was 87-140. There was a distribution of single (rtN204V, rtM204I) and double (M204V+L180M, M204I+L180M, M204V/I+L180M) mutants across the various dose groups.
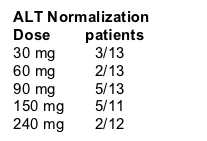
Patients in dose groups 30 mg (n=13/13), 60 mg (n=13/14), 90 mg (n=13/14) completed the study. None of the 12 patients in the 150 and 240 mg groups completed the study yet. There was 1 early withdrwal from each of the 60, 90 & 150 mg groups for high ALT at screening, low platelet at screening, and low ALT at screening, respectively.
Adverse events
There were no serious adverse events as of this presentation. There were no AEs reported that were said to be drug-related except for 2 in the 240 mg group (headache: mild intensity; fatigue: moderate intensity).
|
| |
|
 |
 |
|
|