 |
 |
 |
| |
Interim (Week 12) Phase 2B Virological Efficacy and Safety Results of Albuferon (albumin interferon alfa-2b) Combined with Ribavirin in Genotype 1 Treatment-naive Chronic Hepatitis C Infection
|
| |
| |
Reported by Jules Levin
EASL, April 26-30, 2006, Vienna, Austria
Study authors: S. Zeuzem, Y. Benhamou, D. Shouval, V. Bain, S. Pianko, R. Flisiak, M. Grigorescu, V. Rehak, E. Yoshida, K. Kaita, C. Hezode, A.U. Neumann, M. Subramanian, J. McHutchison
Saarland University, Germany; Hopital Pitie-Salpetriere, France; Hadassah University, Israel; University of Alberta, Canada; Monash University, Australia; Medical University of Bialystok, Poland; Spitalul Clinic de Adulti Cluj-Napoca, Romania; Nuselská poliklinika - Remedis, Czech republic, University of British Columbia, Canada; University of Manitoba, Canada; Hopital Henri Mondor, France; Bar-Ilan University, Israel; Human Genome Sciences, USA; Duke Clinical Research Institute, USA
Study sponsored by Human Genome Sciences, IInc..,, Rockviilllle,, MD,, USA
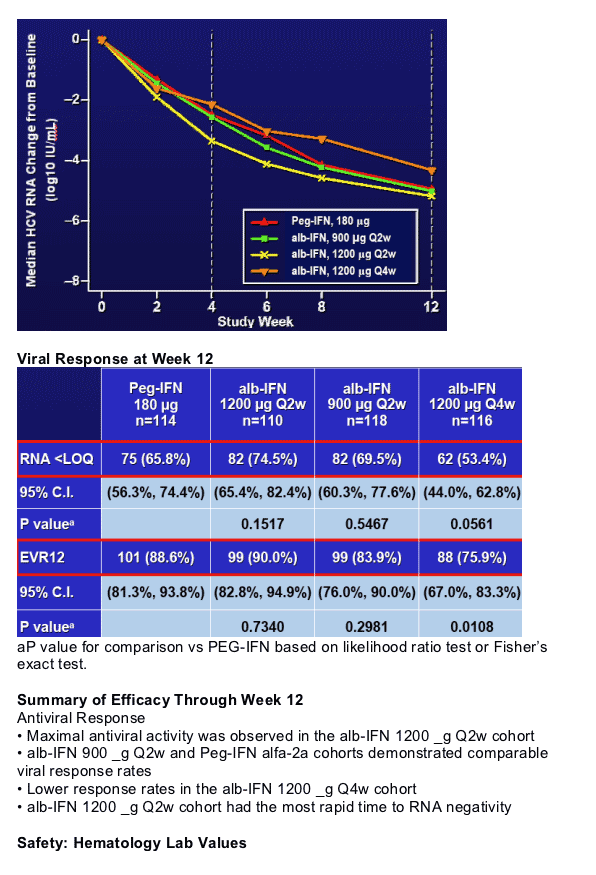
Author Conclusions
- Week 12 Efficacy
- alb-IFN 1200 _g Q2w arm has highest % RNA negative subjects
- alb-IFN 1200 _g Q2w arm has most rapid time to RNA negativity
-- alb-IFN 900 _g Q2w is similar to Peg-IFN alfa-2a
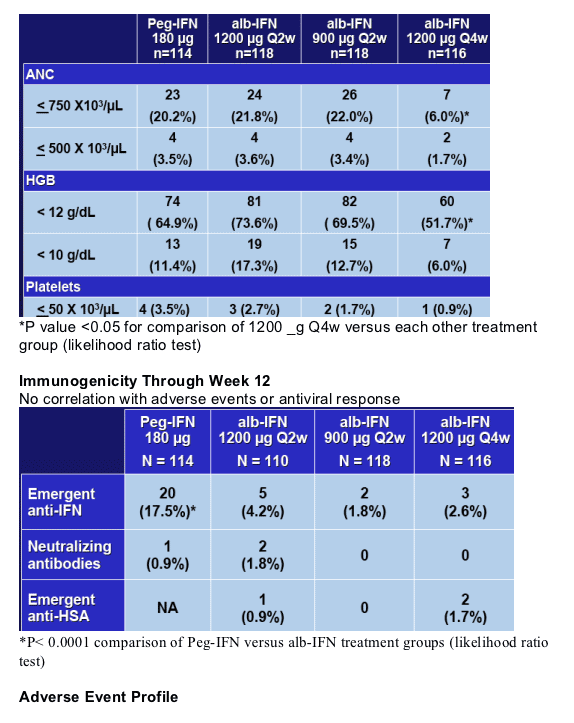
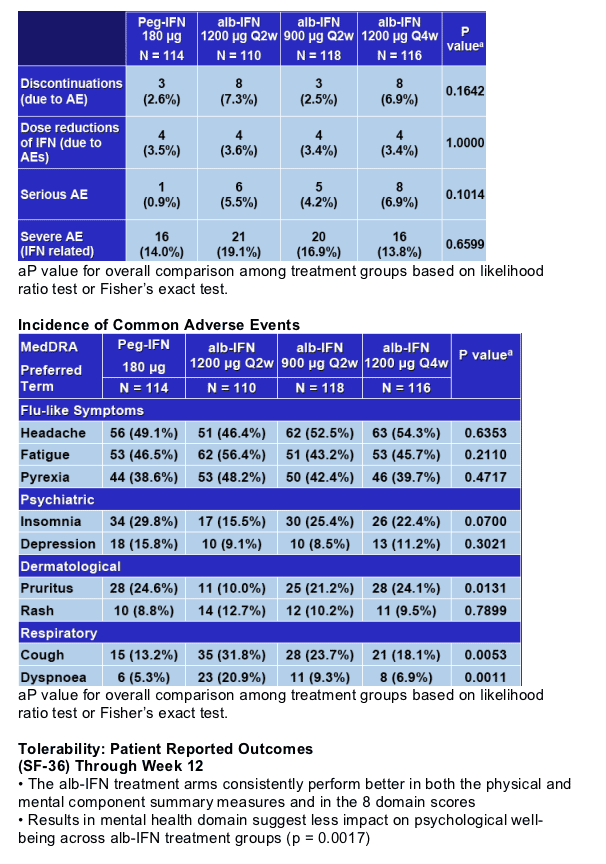
- Week 12 Safety and Tolerability
- Hematology is comparable between alb-IFN Q2w cohorts and Peg-IFN alfa-2a
- Adverse events are generally comparable among all cohorts
- Immunogenicity rates are lower in all alb-IFN cohorts
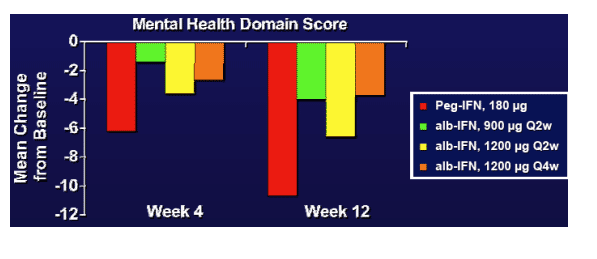
- QoL was impacted less in the alb-IFN cohorts
These data suggest that alb-IFN may offer efficacy and safety at least comparable to Peg-IFN alfa-2a with an improved dosing schedule. These data warrant exploring the alb-IFN Q2w doses in Phase 3.
Once monthly dosing at higher dosing levels are planned to be studied.
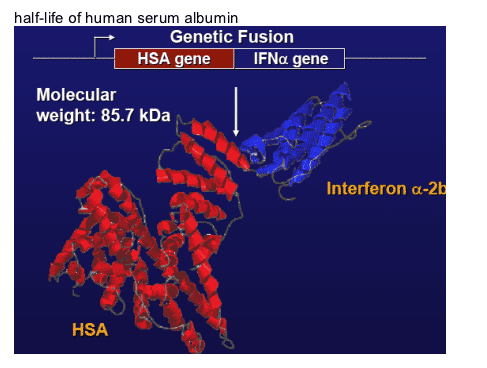
Molecular Model of
Albuferon (albumin interferon alfa-2b)
Albuferon, also called albumin interferon alfa-2b (alb-IFN), is a single polypeptide molecule that combines the therapeutic activity of interferon alpha with the long half-life of human serum albumin
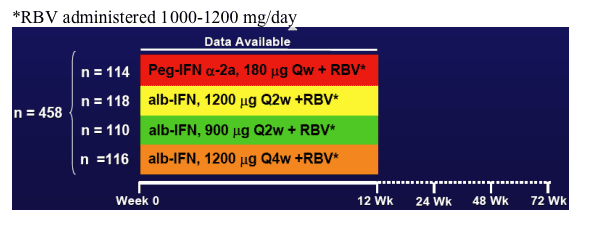
Study Design
- Design: Randomized, open label, active control
- Population: IFN_ treatment-naive, genotype 1
- Conducted at 82 sites in Canada, Europe, Israel and Australia
- 4 cohorts stratified by
- HCV RNA (<800,000 IU/mL, > 800,000 IU/mL)
-- BMI (< 25 kg/m2, >25 kg/m2)
*RBV administered 1000-1200 mg/day
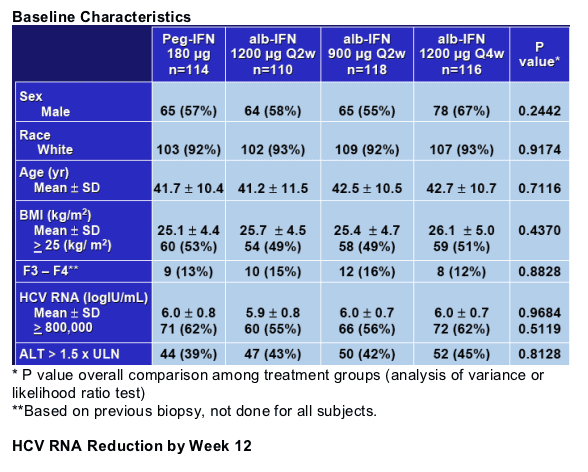
End Points
Primary
- Sustained Virological Response (undetectable HCV RNA at 24 weeks follow up)
Secondary
- Early Virological Response at week 12
- Safety and Quality of Life at week 24 and 48
All analysis presented are ITT (intent to treat)
Laboratory Methods
- HCV RNA: Real-time PCR assay, Quantasure (Labcorp)
- Dynamic range: 43 IU to 69 million IU/mL
- Limits of quantitation: 43 IU/mL
- Limits of detection: 10 IU/mL
- Genotyping: Direct sequencing of the 218 bp 5' non translated region of the HCV genome
|
| |
|
 |
 |
|
|