 |
 |
 |
| |
TMC125 Controversial Response in Early Study
|
| |
| |
8th International Congress on Drug Therapy in HIV Infection
November 12-16, 2006
Glasgow, Scotland
Mark Mascolini
TMC125, an investigational nonnucleoside (NNRTI) with activity against NNRTI-resistant HIV-1, did a poor job of controlling viral replication in a phase 2 study that enrolled people who one or more NNRTI mutations and numerous NRTI mutations. [1]. Viral load declined by a mean 1.3 log at week 8 in patients receiving TMC125 plus 2 NRTIs and then viral load rebounded back up towards baseline. But a poor background therapy, high rates of nucleoside (NRTI) and NNRTI resistance in the study population appear to explain the disappointing result.
Researchers in South Africa, Thailand, Argentina, Brazil, Mexico, Russia, Spain, and the UK randomized 57 people to an investigator-selected protease inhibitor (PI) plus two NRTIs and 59 people to 800 mg of TMC125 twice daily plus two NRTIs. (A new TMC125 formulation achieves the same drug exposure as that dose with 200 mg twice daily.) Everyone had NNRTI experience and at least one NNRTI-linked mutation, but no one had PI experience. Almost all patients took a ritonavir-boosted PI, 61% getting lopinavir/ritonavir and 32% atazanavir/ritonavir. Researchers picked NRTIs based on resistance test results.
Only 20.3% in the TMC125 arm and 22.8% in the PI arm had no NRTI-related mutations when the trial began. About half of those randomized to TMC125 had two or more NRTI mutations at that point, as did about 40% of people randomized to a PI. Many study participants recycled previously used NRTIs in this trial.
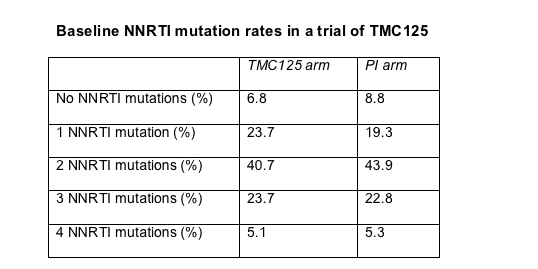
Prerandomization rates of NNRTI mutations were even higher (Table). More than 90% of the study group had at least one NNRTI mutation, and about 70% had two or more. Median fold change in susceptibility measured 129.8 for efavirenz, 88.0 for nevirapine, and 2.0 for TMC125. Presenting these results, Tibotec's Brian Woodfall speculated that many study participants had continued their NNRTI regimen long after virologic failure and thus piled up multiple mutations. Statistical analysis showed that the more NRTI mutations a person had when the trial began, the more NNRTI mutations they had at that point.
In the first 2 weeks of treatment, average viral loads dropped about 1.3 log copies/mL (more than 10-fold) in both treatment groups. But after that the average load in the PI-treated group continued sliding past 2 log (100-fold), while most people taking TMC125 had a viral rebound by week 16. Seeing these results, an independent safety panel recommended switching everyone taking TMC125 to a PI.
Virologic response to the NNRTI was particularly poor in Thailand and South Africa, where study participants had high baseline rates of resistance to NNRTIs and NRTIs. Over 70% of enrollees from those countries had two or more NNRTI mutations when the study began, and about 40% had three or more NNRTI mutations. Subgroup analysis also showed that people with four or more thymidine analog mutations (TAMs) or M184V had almost no response to the TMC125 regimen, while people with two to three TAMs or M184V had an initial response and a sharp rebound.
Even people who started the study with only one NNRTI mutation often endured a rebound after week 8, probably because most had NRTI mutations when the trial started. In a multivariate analysis adjusted for study-entry NRTI and NNRTI resistance, the critical K103N or Y181C mutation at baseline did not predict virologic response among people taking TMC125.
Central nervous system and psychiatric side effects did not differ between the TMC125 group and the PI group. While 10% taking TMC125 had rash, no grade 3 or 4 rashes flared and researchers spotted no gender difference in rash incidence. Gastrointestinal upsets proved less common with TMC125 than with a PI. Diarrhea incidence, for example, measured 3% with TMC125 and 25% in the PI arm. Overall rates of grade 3 or 4 side effects were comparable in the two groups--10% with TMC125 and 12% with a PI. Lipid and bilirubin elevations proved less common with the NNRTI than with a PI.
Woodfall noted that the results underscore the importance of stopping a failing NNRTI regimen as soon as possible to prevent accumulation of mutations. The trial demonstrates that a different strategy to using TMC125 will be required than simply giving it plus 2 NRTIs to people who already have multiple NRTI and NNRTI mutations.
Reference
1. Woodfall B, Vingerhoets J, Peeters M, et al. Impact of NNRTI and NRTI resistance on the response to the regimen of TMC125 plus two NRTIs in study TMC125-C227. 8th International Congress on Drug Therapy in HIV Infection, November 12-16, 2006, Glasgow. Abstract PL5.6.
|
| |
|
 |
 |
|
|