 |
 |
 |
| |
Maraviroc, Changes in CD4 & Tropism in ART-EXperienced 1029 Study
|
| |
| |
Reported by Jules Levin
Eighth International Congress on Drug Therapy in HIV Infection, Glasgow, UK, 12th-16th November 2006
"....An X4 tropism result at failure on MVC was not associated with CD4 decline.... Neither baseline CD4 cell count nor baseline viral load was associated with an X4 tropism result at failure....
.... In contrast, the two patients on placebo + OBT who had an X4 tropism result at failure were clear outliers, having a low baseline VL (mean =3.98 log10 RNA
copies/ml) and high baseline CD4 cell count (384 and 650 cells/mm3). These patients eXperienced a large drop in CD4 counts between baseline and failure...." See tables below
"Selective Suppression of R5-tropic HIV-1 by the Novel CCR5 Antagonist, Maraviroc (MVC), is Associated with Maintenance of CD4 Cell Counts in Antiretroviral-EXperienced Patients Infected with Dual/MiXed-tropic HIV-1"
Elna van der Ryst,1 Howard Mayer,2 James M. Goodrich,2 Karen Turner,1
John F. Sullivan,1 Paul Simpson,1 and Mike Westby1
Pfizer Global Research and Development, 1Sandwich, UK, and 2New London, USA
Poster No. P393
AUTHOR SUMMARY & CONCLUSIONS
Both MVC treatment groups had a higher CD4 cell increase compared with placebo, irrespective of treatment response, indicating that CD4 increases on MVC were not restricted to patients that were on study drug at Week 24.
In patients whose virus was assigned as D/M at screening, 11% had a different tropism result at baseline. This suggests that the proportion of R5, R5X4 and X4 viruses (within a single patient) may vary over time in the absence of selective pressure from MVC.
More patients had an X4 tropism result at Week 24 (or time of failure) in the
MVC arms compared with placebo, consistent with selective suppression by MVC of R5 viruses in these patients.
There was no evidence of an association between baseline CD4 count, viral load or enfuvirtide use and an X4 tropism result at time of failure.
The CD4 increases in the MVC arms did not differ by tropism result at time of failure, confirming that an X4 tropism result was not associated with an adverse CD4 outcome in this patient population.
An X4 tropism result in D/M patients receiving MVC may not have the same clinical consequences as when X4 virus is detected in treatment naive patients.
Introduction & Objectives
HIV-1 strains are categorised according to their co-receptor usage as R5 (CCR5-tropic), X4 (CXCR4-tropic) or R5X4 (dual tropic).1 Patients can be infected with a heterogeneous population of viruses that collectively can infect CCR5 and CXCR4 cells (dual- or miXed tropic [D/M]; see eXplanatory teXt boX).
There is a compleX association between HIV-1 co-receptor tropism, transmission and pathogenesis which is not yet fully understood.
- In approXimately 50% of treatment-naive individuals, X4 strains emerge over time and this has been associated with rapid CD4 T-lymphocyte decline and accelerated disease progression.2
- Whether emergence of X4 strains is a marker for disease progression or the
cause is not known.3,4
- The impact of X4 emergence on disease progression may be different in the conteXt of highly active antiretroviral therapy (HAART).5-9
Maraviroc (MVC), a novel CCR5 antagonist in phase 3 development, is active against R5 strains and has no activity against HIV-1 strains that use CXCR4 (X4 or R5X4 strains).10,11
- It is unclear what effect MVC will have on viral suppression when administered with optimized background therapy (OBT) in patients with D/M HIV-1 (see eXplanatory teXt boX).
Study A4001029 (Figure 1) is an ongoing phase 2b, 48-week, randomized, placebo-controlled trial of MVC (BID and QD) in antiretroviral-eXperienced subjects whose virus at screening is classified as 'non-R5' (X4, D/M or indeterminate).The 24-week results12 indicated that:
- MVC is well tolerated in this patient population
- A similar decrease in viral load was seen in all treatment groups
- Higher mean CD4 count increases were seen in the MVC treatment groups compared with placebo.
The objective of this post hoc analysis was to investigate changes in viral tropism determination in patients with D/M HIV-1 at screening who received MVC in study A4001029, and to determine the potential impact of these changes on clinical outcome.
Study design
- Study design for A4001029 is shown in Figure 1. Patients are treatment-eXperienced (triple-class eXperience and/or documented dual-class-resistant) with at least one active drug in the OBT, HIV-1 RNA >5000 copies/mL, and virus at screening is classified as 'non-R5'.
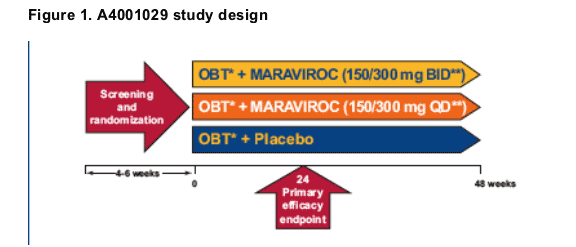
* OBT = 3 to 6 ARVs (excluding low dose RTV)
**A dose of 150 mg was used if MVC was dosed in combination with a PI (excluding TPV/RTV) or delavirdine
Co-receptor tropism testing (Trofile assay, Monogram Biosciences, San Francisco, CA) was performed at the screening and baseline visits for all patients with HIV-1 RNA >500 copies/mL at Weeks 4, 8 and every 8 weeks thereafter (see eXplanatory teXt boX).
As defined in the clinical protocol, tropism testing was cancelled at study visits where VL <500 RNA copies/mL. Accordingly, any tropism results recorded against time-points where VL was <500 RNA copies/mL were censored for this analysis.
Treatment failure was defined as a confirmed:
- increase in HIV-1 RNA at least 3 times the baseline value
- <0.5 log10 HIV-1 RNA decrease from baseline to Week 8
- <1.0 log10 HIV-1 RNA decrease from baseline post Week 8 in patients who previously achieved a >2.0 log10 decrease, or
- Increase to >5000 copies/mL in patients with previously undetectable
HIV-1 RNA.
All randomized patients whose virus was classified as D/M at screening, who met all study inclusion criteria and received at least one dose of study medication were included in this post hoc analysis.
Results are reported according to the regimen the patient received.
For all investigations of CD4 cell counts, a last observation carried forward (LOCF) approach was used to impute missing values.
RESULTS
CD4 cell count increases are seen in patients who fail treatment and those on study drug at Week 24
Mean changes from baseline to Week 24 in CD4 cell count were greater in
the MVC arms compared with the placebo + OBT arm, regardless of treatment response (Table 1).
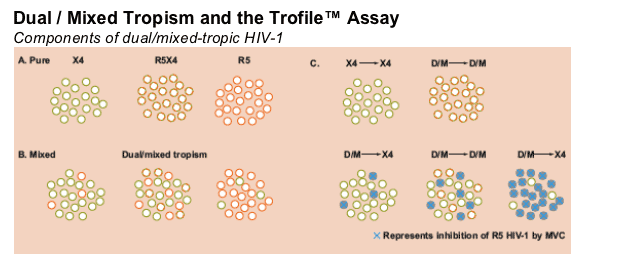
Within a patient, the circulating virus population is composed of various miXtures of closely related but genetically distinct viruses ("quasispecies"). These quasispecies may comprise a pure population that all share the same tropism (panel A). Alternatively, quasispecies with different tropisms may co-eXist in a miXed population (panel B).
The Trofile assay returns the same result (=D/M) when testing the dual-tropic population (R5X4) in panel A or any of the three miXed populations in panel B.
MVC selectively inhibits R5 viruses. The Trofile assay may then return an X4 result in some miXed populations but not others (panel C).

*Assay validated to 1000 copies/mL although in this study samples containing ≥500 copies/mL were tested
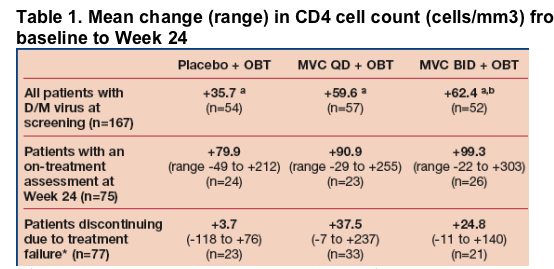
Fifteen patients discontinued treatment prior to Week 24 for reasons other than treatment failure. Data shown are for patients for whom CD4 values were available. aAdjusted mean from ANCOVA model, adjusting for randomization strata. bP<0.05 compared with placebo arm. *Mean time (days) to treatment failure in patients was; placebo 68; MVC QD 69; MVC BID 82.
Changes in viral tropism detection during the study
A change in tropism detection was observed in 18/167 (11%) of patients between screening and baseline study visits.
- 16 patients had virus that went from D/M at screening to R5 at baseline.
- 2 patients had virus that went from D/M at screening to X4 at baseline.
On-treatment suppression of HIV-1 meant that viral tropism could not be assigned to many patients at planned study visits.
- 44/75 (59%) patients on treatment at Week 24 had VL <500 copies/ml
(Table 2).
- 25 patients had VL <500 copies/ml at all planned visits to Week 24.
Of the patients still on treatment at Week 24, virus in 7/75 (9%) patients had changes in tropism detection between screening and week 24 (Table 2).
- Virus in 7/27 (26%) patients with a valid tropism result at Week 24, had changed assignment.
- 5 patients had virus that went from D/M at screening and baseline to X4 at Week 24.
- 2 patients had virus that went from D/M at screening and baseline to R5 at Week 24.
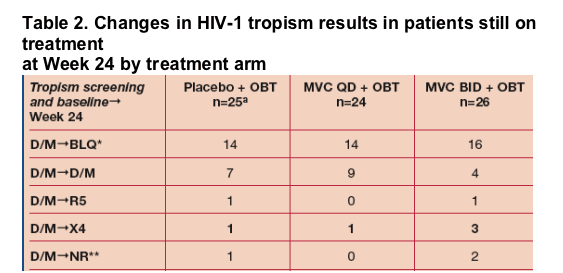
aOne patient in the placebo arm had a missing tropism result at Week 24.
*Below Limit of Quantification (tropism testing censored/cancelled at VL<500 copies/mL)
**No tropism result could be assigned.
Of the patients who discontinued due to treatment failure, virus in 31/75 (41%) patients was assigned a different tropism result at time of failure (Table 3).
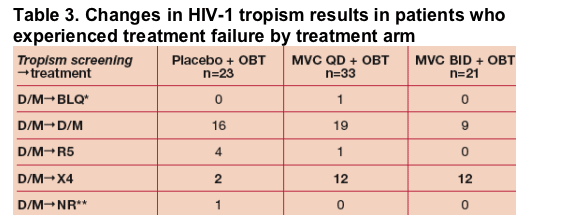
* Below Limit of Quantification (tropism testing censored/cancelled at VL<500 copies/mL)
**No tropism result could be assigned.
Correlation between changes in tropism and markers of clinical outcome
Neither baseline CD4 cell count nor baseline viral load was associated with an X4 tropism result at failure (Table 4).
An X4 tropism result at failure on MVC was not associated with CD4 decline.
- CD4 increases in these patients were greater than in any group of patients on placebo + OBT regardless of tropism determination.
In contrast, the two patients on placebo + OBT who had an X4 tropism result at failure were clear outliers, having a low baseline VL (mean =3.98 log10 RNA copies/ml) and high baseline CD4 cell count (384 and 650 cells/mm3). These patients eXperienced a large drop in CD4 counts between baseline and failure.
There was also no correlation between an X4 tropism result at time of treatment failure and use of enfuvirtide, although the numbers were low (data not
shown).
Table 4. Mean baseline CD4, baseline VL and change in CD4 cell
count at time of failure, by tropism result at failure
For the 2 patients who experienced tropism switch from D/M to X4 mean delta-CD4 loss was -104 (-118 to -89), but for patients on Maraviroc who experienced switch from D/M to X4 mean delta-CD4 increase was +48 (+2 to +164) on MVC QD (n=12) and mean delta-CD4 increase was +33 (-9 to +140) on MVC BID (n=12).
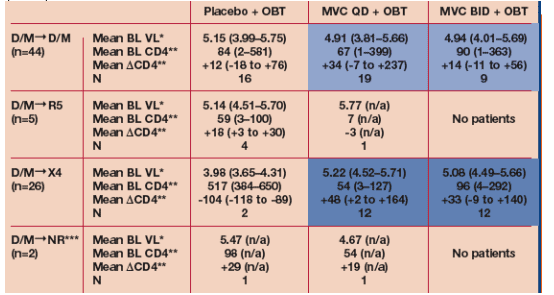
* Mean (HIV-1 RNA log10 copies/mL). ** Mean (cells/mm3). ***No tropism result could be assigned.
Baseline viral load and CD4 count and change in _CD4 at failure were compared between DM to DM and DM to X4 using a 2 sample t-test, combining maraviroc groups (as indicated in coloured boxes), assuming equal variances.
All comparisons were non significant (P>0.05).
References
1. Berger EA et al. Nature 1998;391:240.
2. Schuitemaker H et al. J Virol 1992;66:1354-60.
3. Moore JP et al. AIDS Res Hum Retroviruses;20:111-126.
4. Philpott SM. Curr HIV Res 2003;1:217-227.
5.Waters L et al. 46th ICAAC 2006; Abstract H-1667.
6. Hunt PW et al. J Infect Dis 2006;194:926-30.
7. Delobel P et al. J Acquir Immune Defic Syndr 2005;38:382-92.
8. Melby T et al. J Infect Dis 2006;194:238-46.
9. Philpott S et al. J Clin Invest 2001;107:431-8.
10. Dorr P et al. Antimicrob Agents Chemother 2005;49:4721-32.
11. Fatkenheuer G et al. Nat Med 2005;11:1170-2.
12. Mayer H et al. XVI IAC 2006; Abstract THLB0215.
13. Limoli K et al. XVI IAC 2006; Abstract THPE0045.
|
| |
|
 |
 |
|
|