 |
 |
 |
| |
TMC114 PK & Drug Interactions
|
| |
| |
Reported by Jules Levin
8th Intl Congress on Drug Therapy in HIV
Glasgow, Uk, Nov 12-16, 2006
"Use of darunavir (DRV; TMC114) in combination with other
drugs: guidance from pharmacokinetic studies"
D Back,1 V Sekar,2 E Lefebvre,2 M De Pauw,3 E De Paepe,3 T Vangeneugden,3 R Hoetelmans3
1University of Liverpool, Liverpool, UK, 2Tibotec Inc., Yardley, PA, USA, 3Tibotec BVBA, Mechelen, Belgium
David Back is Professor of Pharmacology at the University of Liverpool and established the Liverpool HIV Pharmacology Group in 1987. The Liverpool group has numerous ongoing pharmacokinetic (TDM, IQ, drug-drug interactions, pharmacological mechanisms of resistance) and pharmacogenomic (phenotype-genotype) studies. They also run the highly successful website www.hiv-druginteractions.org and have recently joined with colleagues in Vanderbilt and Lausanne to launch www.hiv-pharmacogenomics.org.
OVERVIEW OF THIS PRESENTATION
Mode of action and characteristics of DRV
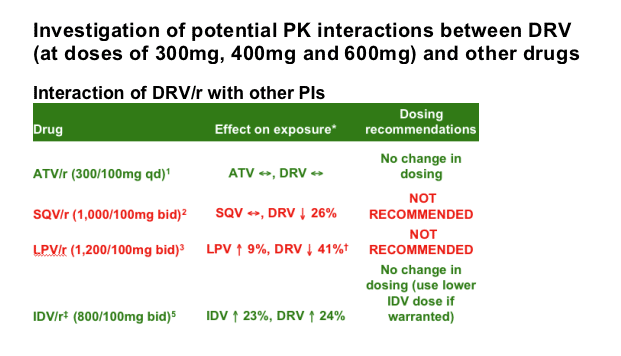
Investigation of potential pharmacokinetic (PK) interactions between DRV (at doses of 300mg, 400mg and 600mg) and other drugs
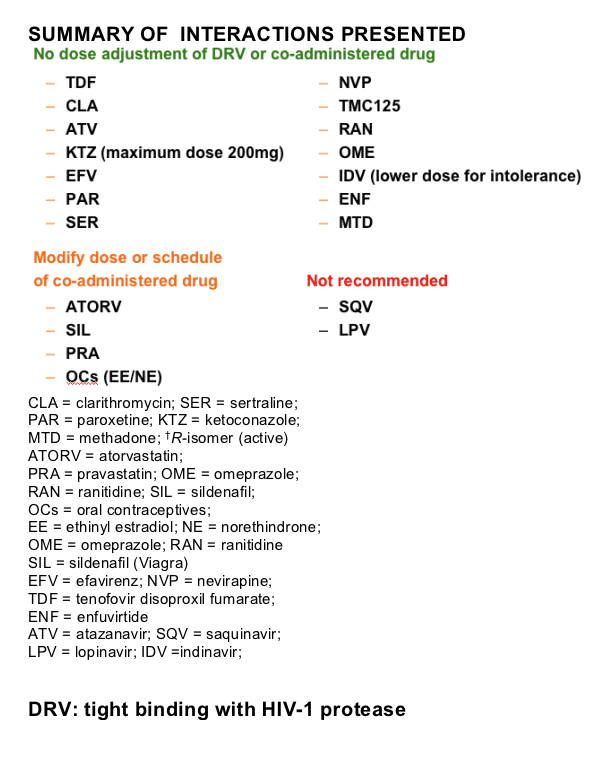
Darunavir (DRV; TMC114) binds tightly with HIV-1 protease; rapid, high-affinity binding is followed by very slow dissociation, which predicts high-virological efficacy and high-genetic barrier to resistance.
HIV-1 protease has an active site (like a pocket with a specific shape). The protease inhibitors fit the pocket and prevent the substrate from interacting at the active site - this is referred to as competitive inhibition. The stability of this inhibition is determined by how many interactions a drug can establish in the active pocket. If a drug has a few interactions, one or two changes in the pocket can displace the inhibitor. DRV binds particularly tightly because it fits the pocket in a similar way to the substrate and has a large number of interactions in the pocket. Even with a number of changes to the pocket, DRV sticks to the pocket and is difficult to displace.
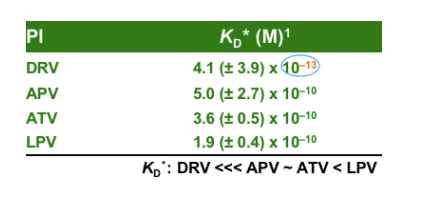
DRV has rapid, high-affinity binding with HIV-1 protease, followed by very slow dissociation. DRV binds faster to protease than other PIs (except APV, which shows an association rate similar to DRV) (kon: APV ∼ DRV > LPV > IDV > RTV > ATV > SQV ∼ NFV). The very high affinity of DRV compared with the other available PIs is almost completely accounted for by the very slow dissociation from wild-type protease which results in a very tight binding (KD: DRV <<< APV ∼ ATV < LPV < NFV < IDV ∼ RTV ∼ SQV)1. This predicts a high virological efficacy and high genetic barrier to resistance. The parameters determined for the currently available PIs are in line with published results (ref KD).2
References
1. Dierynck I, et al. 14th IHDRW 2005. Abstract 64.
2. Shuman CF. J Mol Recognit 2004;17:106-19.
Abbreviations
APV = amprenavir
ATV = atazanavir
IDV = indinavir
LPV = lopinavir
NFV = nelfinavir
PI = protease inhibitors
SQV = saquinavir
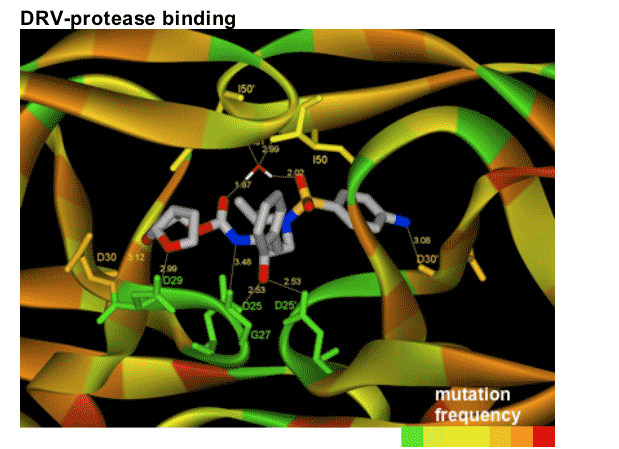
In this figure, the numbers represent the distance in _ngstroms between DRV and the amino acids that make up the protease structure. It can been seen that these numbers are very small indicating that DRV fits neatly in the protease pocket. Therefore the structure is stable and the protease enzyme cannot easily remove DRV from the pocket.
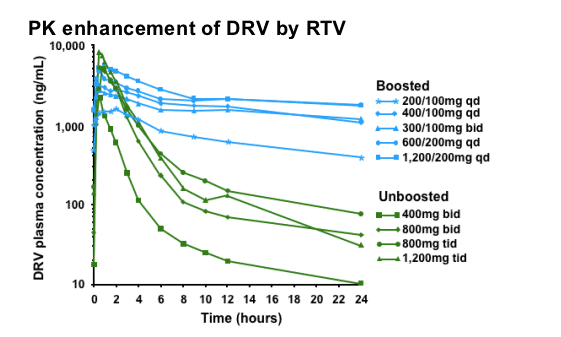
The addition of 100mg RTV to DRV increases the half-life and therefore enhances the pharmacokinetics (PK) of DRV.
This slide demonstrates the impact of boosting DRV with RTV. The effect of different regimens (boosted and unboosted) on plasma concentrations of DRV is illustrated. It is clear that the inclusion of low-dose RTV (100mg) in the regimen has a beneficial impact on DRV half-life. Note: this graph does not show the recommended regimen of DRV/r 600/100mg twice daily. Also note that the y-axis is a logarithmic scale.
Reference
Hoetelmans R, et al. 10th CROI 2003. Abstract T39 and poster 549.
The bioavailability of DRV is increased when taken with food
Intake with food increases the bioavailability of DRV versus fasted state. DRV/r should be taken with food, and the type of food does not affect systemic exposure to DRV/r. From a practical standpoint, it is important to consider the effect of food on DRV PKs. Interestingly, the type of food is not important - there is a roughly 30% increase in the bioavailability of DRV regardless of the type of food eaten. It is recommended therefore that DRV be taken with food.
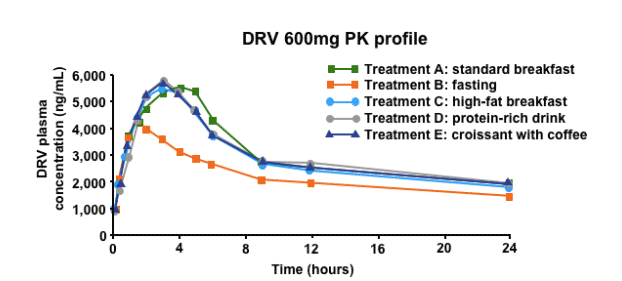
References
1. Hoetelmans R, et al. 5th IWCPHIV 2003. Poster P5.6.
2. Sekar V, et al. 10th EACS 2005. Abstract PE4.1/1.
PK profile of DRV: summary
-- Co-administration with low-dose RTV improves PK1
-- Should be taken with food2
-- Elimination half-life (12-15 hours) supports daily dosing
--Metabolism is CYP3A4 mediated; also role of P glycoprotein
-- In-vitro plasma glycoprotein (AAG) binding similar to other PIs3
-- In-vivo plasma protein binding >95% (mostly AAG) (AAG = alpha 1-acid glycoprotein)
-- Lack of PK-PD (PD=pharmacodynamic) relationship for efficacy and safety at the recommended dose of 600/100mg bid in treatment-experienced HIV-infected patients.4
Co-administration of DRV with low-dose RTV improves its PK profile (approximately 14-fold increase in exposure compared to unboosted DRV).1 DRV should be taken with food.2 In the presence of low-dose RTV, systemic exposure to the drug is increased by ∼30% after feeding with a standard meal compared with fasting. The type of meal does not affect exposure to DRV. Plasma concentrations of DRV are similar for men and women after feeding with a standard meal. DRV has a long elimination half-life of 12-15 hours, which makes it suitable for both once and twice daily dosing. Like the other PIs, DRV is highly (96%) bound to plasma proteins (particularly alpha 1-acid glycoprotein).3 Its clearance involves oxidative hepatic metabolism mediated by cytochrome (P450) CYP3A4, and P glycoprotein also has a role; this means that drug interactions should be considered.
References
1. Hoetelmans R, et al. 12th CROI 2003. Abstract 549.
2. Sekar V, et al. 10th EACS 2005. Abstract PE4.1/1.
3. de Bethune MP, et al. 9th CROI 2002. Abstract T-39.
1. Sekar V, et al. 10th EACS 2005. Abstract PE4.3/4
2. Sekar V, et al. 44th IDSA 2006. Poster P959
3. Sekar V, et al. 46th ICAAC 2006. Abstract A-0367
4. Tibotec BVBA, Mechelen, Belgium. Data on file
5. Sekar V, et al. 14th ISHEID 2006. Abstract PP4.15
Despite a two-fold increase in DRV dose (from 600mg to 1,200mg bid), exposure to DRV was reduced by 41% in HIV-infected patients when co-administered with LPV/r vs DRV/r alone (in a previous study when LPV/r 400/100mg was used, DRV exposure was reduced by 53% in HIV-negative healthy volunteers4)
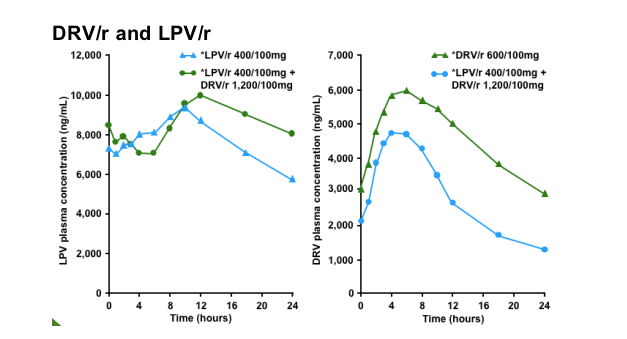
LPV steady-state concentrations were generally reached within 5 days of dosing for the different treatments. At steady-state, co-administration of LPV/r 400/100mg bid and DRV/r 1,200/100mg bid resulted in LPV plasma concentrations similar to those observed after administration of LPV/r 400/100mg bid alone. At steady-state, co-administration of DRV/r 1,200/100mg bid and LPV/r 400/100mg bid resulted in lower plasma concentrations of DRV than after administration of DRV/r 600/100mg bid alone.
Reference
Sekar V, et al. 46th ICAAC 2006. Abstract A-0367.
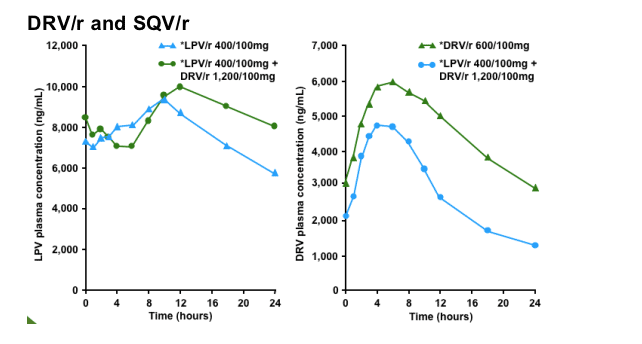
Steady-state concentrations of SQV were achieved prior to PK sampling on Day 14. Mean plasma concentrations of SQV were slightly lower when SQV/r was co-administered with DRV than when administered alone.
Reference
Sekar V, et al. 44th IDSA 2006. Abstract 959.
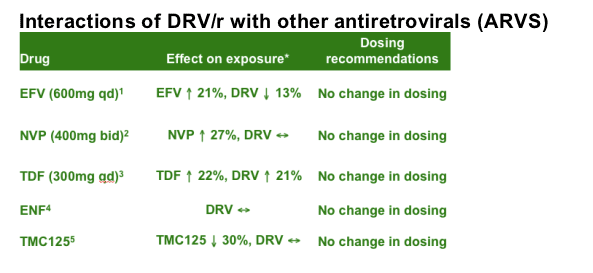
Interaction studies have shown that DRV/r can be combined with the ARVs efavirenz (EFV), nevirapine (NVP), tenofovir disoproxil fumarate (TDF) or enfuvirtide (ENF) without a change in the dosing of either agent.
Studies have been conducted on the interaction of DRV/r with other ARVs. A study of the interaction between DRV/r and the non-nucleoside reverse transcriptase inhibitor (NNRTIs) EFV and NVP indicates that there are small changes in the exposure to both drugs; therefore, no change in the dosing of either agent is needed.1,2 The debate about the combination of TDF and boosted PIs continues; an increase of about 20-30% in TDF levels is always observed. The combination of TDF and DRV/r is accompanied by an increase in TDF levels of 22% (this is comparable to the interaction seen between LPV/r and TDF), plus an increase in DRV levels of 21%; however, current recommendations are that no change in dosing is needed for this combination.3 There are substantial data on TDF-DRV co-administration in the POWER studies. There is no increased exposure to DRV when ENF and DRV/r are combined, therefore no dose adjustment is needed.4 There is no increased exposure to DRV when TMC114 and DRV/r are combined, therefore no dose adjustment is needed.5
References
1. Sekar V, et al. 7th IWCPHIV 2006. Poster P55.
2. Sekar V, et al. 44th IDSA 2006. Poster P956.
3. Hoetelmans R, et al. 15th IAC 2004. Abstract TuPeB4634.
4. Sekar V, et al. 7th IWCPHIV 2006. Poster P54.
5. Boffito M et al. 13th CROI 2006. Abstract 575C.
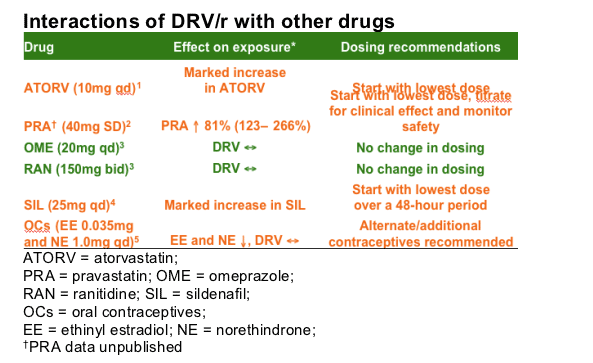
Interaction studies have shown no dose changes necessary when combining DRV/r with omeprazole (OME) or ranitidine (RAN), whereas a dose change is required with atorvastatin (ATORV) and sildenafil (SIL). Alternative/additional contraceptives to estrogens (estradiol and norethindrone) are recommended when DRV is co-administered. When using DRV/r with pravastatin (PRA), start with the lowest dose of PRA, and titrate it up to the desired clinical effect while monitoring for safety.
Studies have been conducted on the interaction of DRV/r with other drugs, such as the statins, oral contraceptives, acid-reducing agents and drugs used to treat erectile dysfunction. A study of the interaction between DRV/r and ATORV indicates that there is a marked increase in the concentration of ATORV and dosing recommendations are that the lowest dose of ATORV (10mg) should be employed initially if this combination is used.1 As ATORV metabolism is CYP3A mediated, this marked increase in ATORV levels is expected, and is presumably driven by the RTV component of DRV/r. Of the statins, PRA is usually not affected by drug interactions. However, the combination of DRV/r and PRA results in a variable change and sometimes large increase in PRA levels.2 Based on these data, when using the combination of DRV/r-PRA, it is recommended to start with the lowest dose of PRA, and titrate the dose up to the desired clinical effect while monitoring for safety. A study of the interaction between DRV/r and OME indicates that there is no change in DRV levels, and no change in the dosing of either agent is needed.3 Similarly, a study of the interaction between DRV/r and RAN indicates that there is no change in DRV levels, and no change in the dosing of either agent is needed.3 A study of the interaction between DRV/r and SIL found exposure to SIL increased four-fold when used in combination with DRV/r.4 A study of the interaction between DRV/r and estrogen found a 44% decrease in estrogen exposure when DRV/r was co-administered; additional contraception is therefore required.5
References
1. Hoetelmans R, et al. 44th ICAAC 2004. Abstract H-865.
2. Tibotec BVBA, Mechelen, Belgium. Data on file.
3. Sekar V, et al. 6th IWCPHT 2005. Abstract 2.10.
4. Sekar V, et al. 46th ICAAC 2006. Abstract A-0369.
Sekar V, et al. 46th ICAAC 2006. Abstract A-0368.
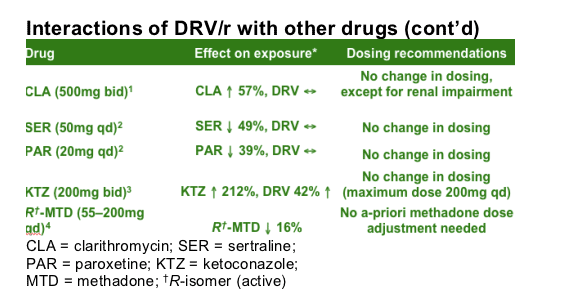
Interaction studies have shown no dose changes are necessary when combining DRV/r with clarithromycin (CLA) or methadone (MTD), or the mood disorder drugs, sertraline (SER) and paroxetine (PAR), or the anti-fugal agent ketoconazole (KTZ).
A study of the interaction between DRV/r and CLA found exposure to CLA was increased by 57% when co-administered with DRV; exposure to DRV was not affected.1 For patients with a creatinine clearance rate of 30-60mL/min and <30mL/min, the dose of CLA should be reduced by 50% and 75%, respectively. A study of the interaction between DRV/r and PAR or SER found a decrease in exposure to PAR or SER by 39% and 49%; DRV exposure was not affected.2 Dose titration of SER or PAR is recommended. More detail on SER and PAR presented in a poster (P295) at this Glasgow meeting. A study of the interaction between DRV/r and KTZ found exposure to KTZ was increased by 212% when co-administered with DRV; exposure to DRV was increased by 42%.3 When these drugs are used in combination, the maximum daily dose is KTZ 200mg. A study of the interaction between DRV/r and MTD where the dose was individualised found a decrease in MTD by 16% (R-isomer [active]) to 36% (S-isomer).4 No a-priori adjustment of MTD dosage is required when MTD and DRV/r are co-administered. More detail on MTD presented in a poster (P294) at this Glasgow meeting.
References
1. Sekar V, et al. ASCPT 2006. Poster PI-61.
2. Sekar V, et al. 8th ICDTHI 2006. Poster P295.
3. Sekar V, et al. 44th IDSA 2006. Poster P960.
Sekar V, et al. 8th ICDTHI 2006. Poster P294.
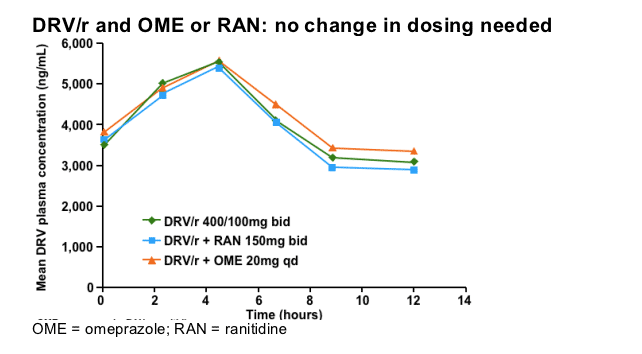
This slide illustrates the lack of interaction when DRV/r is co-administered with the H2-blocker ranitidine (RAN) or the proton pump inhibitor omeprazole (OME). The concomitant use of OME or RAN with DRV/r in HIV-1-infected patients is not expected to result in reduced antiviral effect.
Note: These data are not based on the recommended dose of DRV/r, 600/100mg bid. Also no data are available on higher doses of OME.
Reference
Sekar V, et al. 6th IWCPHT 2005. Abstract 2.10
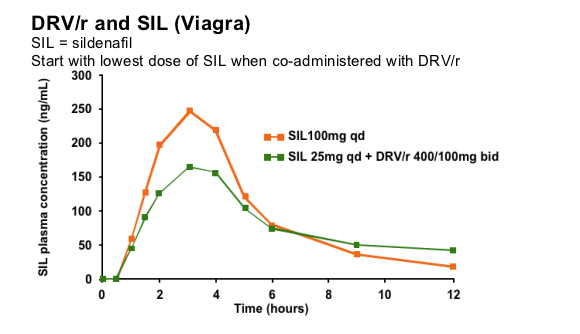
There is an interaction between DRV/r and SIL that results in increased levels of SIL. If the combination is used, the lowest dose of SIL (25mg over 48 hours) should be used initially.
SIL exposure is markedly increased in the presence of DRV/r due to inhibition of CYP3A4. A study in healthy volunteers showed that the low dose of SIL 25mg in combination with DRV/r 400/100mg bid results in a similar exposure compared to SIL 100mg alone. SIL should therefore be initiated at 25mg over a 48 hour period in volunteers receiving DRV/r. The dose could then be increased based upon clinical response.
These findings can be used to provide dose recommendations for other PDE-5 inhibitors that are also CYP3A substrates (vardenafil: single dose not exceeding 2.5mg in 72 hours; tadalafil: single dose not exceeding 10mg in 72 hours) when co-administered with DRV/r.
Note: these data are not based on the recommended dose of DRV/r, 600/100mg bid.
Reference
Sekar V, et al. 46th ICAAC 2005. Abstract A-0369.
|
| |
|
 |
 |
|
|