 |
 |
 |
| |
Trough Concentrations in HIV patients Receiving Boosted Hard-Gel Capsules (HGC) and 500 mg Film-Coated Tablet (FCT) Formulations of Saquinavir
|
| |
| |
Reported by Jules Levin
8th Intl Congress on Drug therapy in HIV, Glasgow, UK, Nov 12-16, 2006
Laura Dickinson1, David Back1, Alan Winston2, Saye Khoo1 and Marta Boffito3
1 Department of Pharmacology, University of Liverpool, Liverpool, UK
2 Imperial College, London, UK
3 PK Research Ltd, Chelsea & Westminster Hospital, London, UK
INTRODUCTION
Management of HIV infection is lifelong therefore development of novel antiretrovirals is crucial in order to control viral replication.
However, improvement of established therapies (e.g. better dosing schedule or formulation) is also important to widen treatment options available to patients.
A new 500 mg film coated tablet (FCT) formulation of saquinavir (SQV) has been developed, lowering pill burden of SQV-containing regimens [compared to 200 mg hard-gel capsules (HGC)].
Single dose SQV FCT is bioequivalent with single dose SQV HGC in healthy volunteers 1.
OBJECTIVES
Compare SQV steady state trough concentrations (C-trough) obtained from patients administered boosted SQV HGC or FCT (SQV/RTV: 1000/100 mg bid).
AUTHOR CONCLUSIONS
Significantly higher SQV C-trough were observed with SQV FCT compared to HGC formulation, which is in agreement with a recent TDM-based study 8.
Improved SQV concentrations achieved with FCT and lower pill burden associated with the RTV boosted regimen are clearly beneficial to patients.
METHODS
Patients: 34 (6 female) HIV infected patients were included from a total of 4 SQV HGC pharmacokinetic (PK) studies 2-5 [median (range) CD4 count and viral load were 367 cells/mm3 (76-970) and 519 copies/ml (57-14580), respectively] and 22 (5 female) from one SQV FCT study 6 [median (range) CD4 count and viral load were 456 cells/mm3 (256-1023) and 132 copies/ml (48-2680), respectively]. Ten patients received both HGC and FCT.
PK assessment: All patients received a standard fat containing meal prior to drug intake.
Ctrough were determined 12 hours post-dose. 5 Ctrough samples were drawn from patients administered SQV FCT on days 7, 10, 13, 17, 20 of a longitudinal study.
SQV/RTV plasma concentrations were determined by HPLC-MS/MS 7.
Data analysis: Differences between SQV HGC and FCT C-trough on each day were assessed by Kruskal-Wallis.
Inter and intra-individual variability in C-trough concentrations were determined by calculation of co-efficient of variation [CV (%); CV = (s.d./mean) x 100].
RESULTS
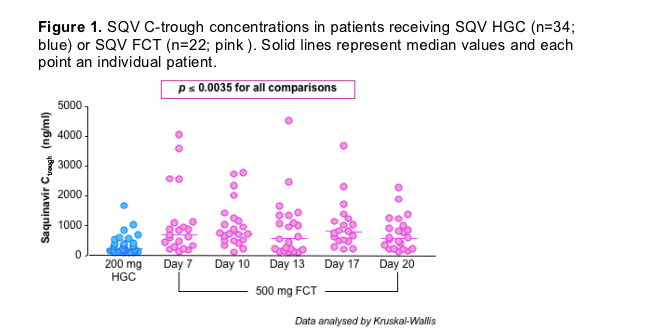
SQV C-trough concentrations from patients administered SQV HGC (n=34) and FCT (n=22) are summarised (Figure 1).
Median (range) SQV Ctrough was 234 ng/ml (73-1663) for HGC.
Median (range) SQV FCT C-trough were 686 ng/ml (128-4041), 735 ng/ml (106-2761), 577 ng/ml (84-4502), 809 ng/ml (197-3668) and 587 ng/ml (134-2262) on days 7, 10, 13, 17 and 20, respectively.
SQV FCT C-trough were significantly higher than HGC C-trough on all 5 days
(p ≦ 0.0035; all comparisons; Kruskal-Wallis).
Both formulations demonstrated marked inter-individual variability in concentrations of 94 % for SQV HGC and 105, 75, 114, 82 and 77 % on days 7, 10, 13, 17 and 20, respectively for SQV FCT.
Ctrough were below the recommended minimum effective concentration for SQV (MEC < 100 ng/ml) in 3/34 and 2/22 (day 13 only) patients administered SQV HGC and FCT, respectively.
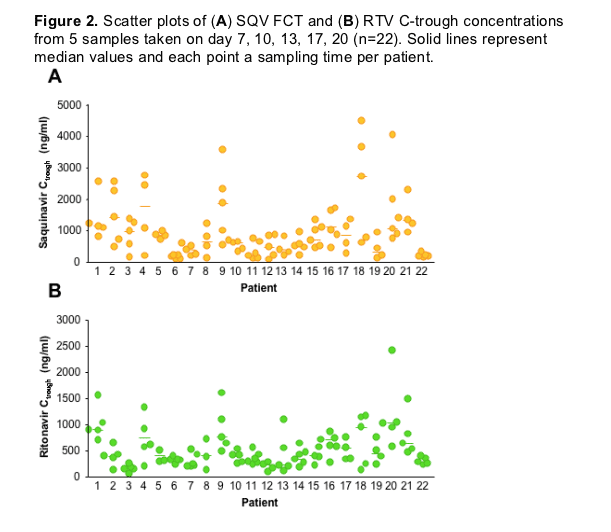
Additionally, intra-individual variability in in both SQV and RTV C-trough concentrations was determined for those patients administered SQV FCT based on 5 C-trough samples (day 7, 10, 13, 17, 20; n=22).
Intra-individual variability in SQV and RTV C-trough are illustrated for each patient (Figure 2).
Median (range) intra-individual variability in SQV C-trough was 54 % (13-90) and 46 % (18-91) for RTV.
REFERENCES
Bittner B. et al. Antivir Ther 2005; 10 (7): 503-10
Boffito M. et al. J Antimicrob Chemother 2005; 55 (4): 542-5
Boffito M. et al. Br J Clin Pharmacol 2005; 59 (1): 38-42
Boffito M. et al. JAIDS 2004; 37 (3): 1376-84
Boffito M. et al. Antivir Ther 2004; 9 (3):423-29
Boffito M. et al. 7th PK Workshop 2006, Lisbon. Abstract 66
Dickinson L. et al. J Chrom B 2005; 829 (1-2): 82-90
Breske A. et al. 7th PK Workshop 2006, Lisbon. Abstract 72
|
| |
|
 |
 |
|
|