 |
 |
 |
| |
Switching to Saquinavir 500-mg Based Regimens in ART-Experienced
|
| |
| |
Reported by Jules Levin
8th Intl Congress in Drug Therapy for HIV
Nov 12-16, 2006
Glasgow, UK
"SWITCHING TO SAQUINAVIR 500 MG-BASED REGIMENS
MAINTAINS ANTIRETROVIRAL EFFICACY"
Mahungu T, Ballinger J, Swaden L, Smith C, Youle M and Johnson M
Royal Free Centre for HIV Medicine, London, UK
AUTHOR CONCLUSION
SQV-500/r-based regimens are well tolerated in both treatment-naive and
treatment-experienced patients.
Switching treatment-experienced patients from the SQV-200 to SQV-500 does not lead to virological rebound or perturbations in serum transaminase or lipid levels.
There were no differences in virological response or tolerability between the
bid and qd regimens.
These findings add to the body of clinical trial evidence supporting the efficacy and safety of boosted SQV-500 in ARV-naive and pretreated patients.
Introduction
Ritonavir-boosted saquinavir (SQV/r, Invirase/r) is recognized as a suitable component of first-line treatment regimens for adults with HIV infection.1,2
SQV has a well-established safety and efficacy profile, and when boosted with saquinavir, has a high barrier to genetic resistance.3
There is also evidence to indicate that SQV/r is effective and well tolerated
when given once daily.4
The new 500 mg film-coated tablet formulation of SQV (SQV-500, Invirase 500) is more convenient for patients than the previous 200 mg formulations of saquinavir (SQV-200) because it reduces the pill burden of SQV/r at the approved dosage of 1000/100 mg bid from six to three pills twice daily.
SQV-500 is also based on the Invirase formulation, which has been shown to have a better tolerability profile when boosted with ritonavir than the Fortovase (soft capsule) formulation of saquinavir.5
Switching from SQV-200 to SQV-500 is appealing because of the lower pill
burden associated with the latter formulation.
Objective
To evaluate the efficacy and tolerability of regimens containing SQV-500.
Study design and patients
All HIV-infected patients attending the Royal Free Centre for HIV Medicine
who had received SQV-500/r before April 2006 were eligible for this retrospective cohort study.
Patient characteristics, disease stage and previous treatment history were
recorded prior to initiating SQV-500-based therapy.
The proportion of patients remaining on SQV-500 after 6, 12 and 24 weeks
was calculated using Kaplan-Meier methods.
Study assessments included plasma HIV-1 RNA, CD4 count, lipid markers
and liver function tests.
Patients were followed until 1 July 2006.
Patient demographics
A total of 237 adults were enrolled in the cohort study.
Demographics and baseline (= at initiation of SQV-500 therapy) characteristics of study participants are summarized in Table 1.
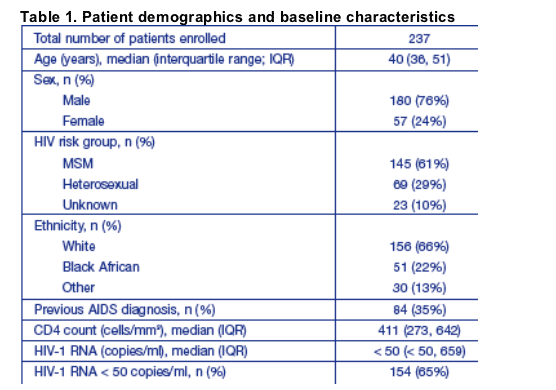
Patients included in this analysis had received SQV-500 for a median of 450 (range 66-744) days. Details of patient treatment history are summarized in Table 2.
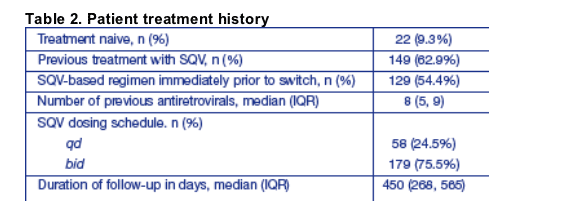
The first patient to receive SQV-500 started therapy in June 2004; the last person included in this analysis started therapy with SQV-500 in April 2006.
Patients received SQV-500-containing regimens that included SQV/r 1000/100 mg bid (n = 179) or 2000/100 mg qd (n = 58).
Among the 149 patients who had previously taken SQV, 129 (87%) switched from ongoing therapy with SQV-200 to SQV-500.
Forty patients have either stopped SQV-500 treatment (n = 19) or modified the dose or schedule of administration (n = 21).
At 6, 12 and 24 weeks, 97.1% (95% CI: 94.9, 100), 96.2% (93.8, 98.6) and 92.1% (88.6, 95.6) of patients remained on SQV-500, respectively.
Efficacy
Viral load (VL) and CD4 count responses to treatment after starting SQV-500 therapy are available for 232 of 237 (97.9%) patients.
Subjects with HIV RNA < 50 copies/ml at baseline
The 152 subjects with HIV RNA < 50 copies/ml at baseline had a median exposure to antiretroviral therapy of 6.9 years (IQR: 3.2-8.8) and had received a median of 8 ARVs (IQR: 6-10).
These patients were followed for a median of 400 days (range 15-686), and had a median of four (range 1-16) VL measurements during this time.
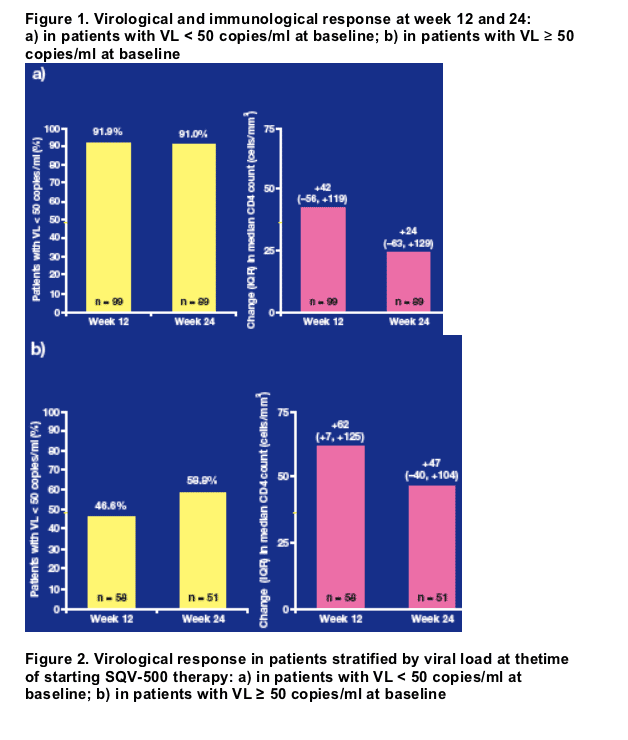
Viral load suppression to < 50 copies/ml was observed in 91.9% and 91.0% of patients after 12 and 24 weeks of treatment, respectively (Figure 1a).
The median CD4 cell counts increased above baseline levels by 42 and 24 cells/mm3 at weeks 12 and 24, respectively (Figure 1a).
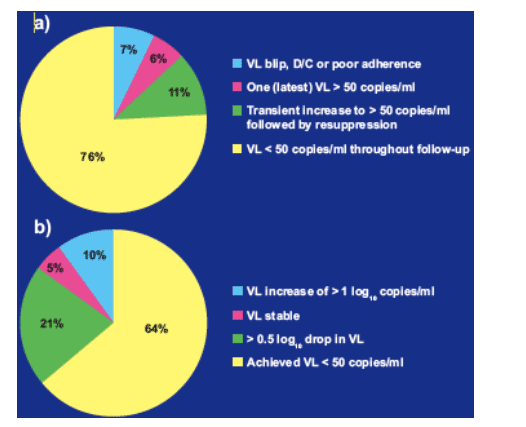
Of the 152 patients with a viral load of < 50 copies/ml at baseline, 132 (87%) experienced successful virological suppression over 24 weeks, with 116 (76%) exhibiting viral loads < 50 copies/ml throughout follow-up and a further 16 patients (11%) experiencing one or more transient increases in viral load followed by resuppression (Figure 2a).
Subjects with HIV RNA ≥ 50 copies/ml at baseline
The 80 subjects with HIV RNA ≥ 50 copies/ml at baseline had a median exposure to antiretroviral therapy of 2.1 years (IQR: 0.1-7.0) and had received a median of 6 ARVs (IQR: 3-9).
27 of the 58 (46.6%) patients with a measurement after 12 weeks of treatment had achieved a viral load < 50 copies/ml; this reached 30/51 (58.8%) at 24 weeks (Figure 1b).
These patients were followed for a median of 255 (range 6-603) days, and had a median of four (range 1-16) VL measurements during this time.
Median CD4 cell counts increased above baseline levels by 62 and 47 cells/mm3 at weeks 12 and 24, respectively (Figure 1b).
Overall, 64% of these patients achieved a viral load < 50 copies/ml during follow-up, with a further 21% achieved a > 0.5 log10 drop in viral load (Figure 2b).
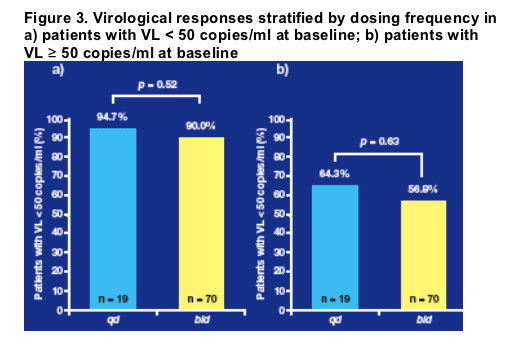
SQV-500 bid vs. qd
A comparison of 24-week virological response rates for SQV-500 bid vs. qd
regimens is shown in Figure 3.
There were no statistically significant differences in the virological response
rates at week 24 between patients treated qd or bid. This finding was consistent among those who had a viral load of < 50 copies/ml at the start of treatment (Figure 3a) or ≥ 50 copies/ml at the start of treatment (Figure 3b).
Safety and tolerability
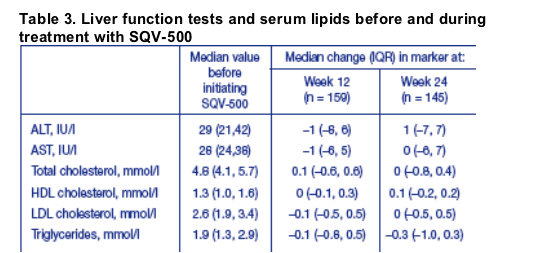
Overall, there were no significant changes in serum transaminase or lipid levels from baseline over the first 24 weeks of treatment (Table 3). There was also no significant differences in these parameters between patients treated with qd or bid SQV/r regimens.
Three patients stopped SQV-500 because of tolerability concerns, two of whom had gastrointestinal symptoms and one of whom had hyperlipidaemia.
No new AIDS-defining events were detected in any patient after initiating treatment with SQV-500/r.
One death occurred that was attributed to a pre-existing AIDS-related malignancy.
|
| |
|
 |
 |
|
|