 |
 |
 |
| |
Kaletra Tablets Improve Tolerability/Diarrhea Compared to Soft-Gel Capsules
|
| |
| |
Reported by Jules Levin
8th International Congress on Drug Therapy in HIV Infection
November 12-16, 2006, Glasgow, UK
Note from Jules Levin: there were several posters reporting on improved tolerability of the new Kaletra tablets compared to the older formulation of soft-gel capsules. The Gathe poster is the only study so far using a validated instrument/questionnaire to assess tolerability. Abbott is planning a larger study to evaluate these questions.
"Tolerability and Therapy Preference of Lopinavir/ritonavir (Kaletra) Soft Gel Capsules and Tablets as Single Agent in a Cohort of HIV Positive Adult Patients (IMANI-2)"
Gathe Jr. JC1, Lipman BA2, Mayberry C3, Miguel B1, Nemecek J3
1Therapeutic Concepts, Houston, TX; 2Abbott Laboratories, 3Donald R. Watkins Foundation, Houston, TX
AUTHOR CONCLUSIONS
This study offers the opportunity to examine LPV/r related tolerability without influences of other antiretroviral agents. Study patients were administered a series of questionnaires immediately prior to and 4 weeks following switch from Kaletra soft-gel capsules to new Kaletra tablet formulation. These include: Medical Outcomes Study - HIV (MOS-HIV); Center for Epidemiologic Studies - Depression (CES-D); Modified Global Condition Improvement (GCI). Prevalence of diarrhea was captured via adverse event reporting prior to and following tablet switch.
Following switch from LPV/r soft-gel capsules to tablets, subjects reported:
-- Significant reduction/elimination of diarrheal adverse events (see table below)
-- Significant improved general health and ability to function in role at work or in the home
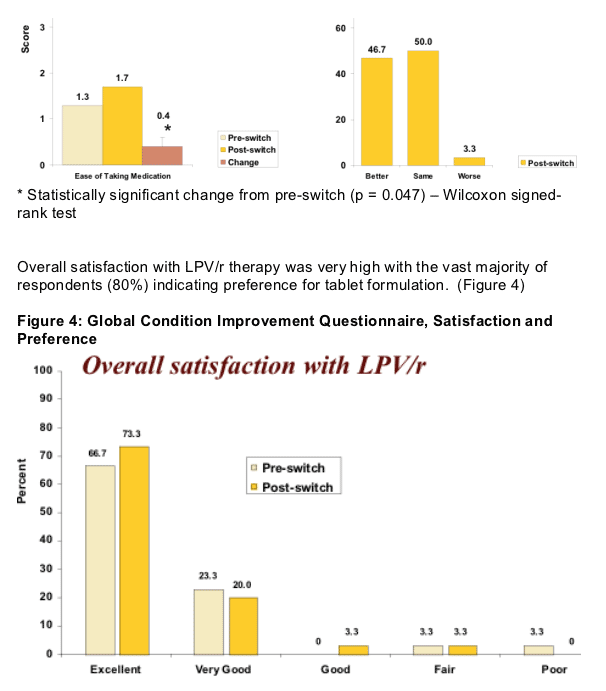
-- Significantly improved self reported ease of taking medication
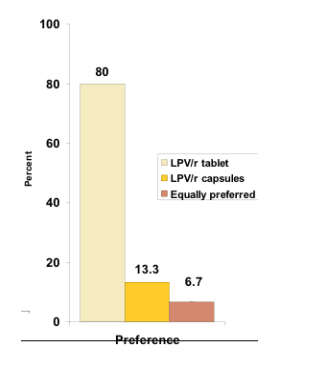
-- High satisfaction and preference for LPV/r in tablet formulation
The improvements in tolerability and preference for LPV/r tablet formulation may improve adherence and virologic and immunologic outcomes.
BACKGROUND
Lopinavir (LPV) is an HIV protease inhibitor that is co-formulated with Ritonavir {r}. The ritonavir acts as a pharmacokinetic enhancer for LPV. The co-formulation is marketed as both Kaletra and Aluvia (LPV/r).
The originally available versions of LPV/r were soft gelatin capsules (SGC) 133/33mg and liquid solution formulation. Limitations of the SGC's include a requirement to be taken with a meal to improve pharmacokinetics. [1,2] Also patients reported diarrhea. Excipients in the SGC including oleic acid, polyethylene glycol, and sorbitol, can be associated with diarrhea. [3] A new formulation of LPV/r received US FDA approval, October 2005. LPV/r tablets 200/50mg employ a novel technique, Meltrex, for drug delivery that does not require the inclusion of many excipients required for the SGC's. Due to improved pharmacokinetics, the tablet formulation can be taken without food.
Studies have been done on LPV/r as triple drug therapy, but there are no studies of single-agent LPV/r tablet tolerability that have been published to date.
METHODS
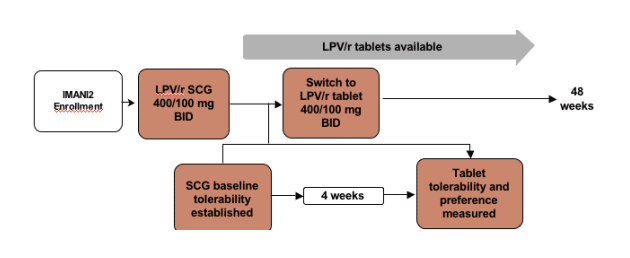
IMANI-2 is a prospective, single center, single-arm, open-label, ongoing trial exploring LPV/r single agent therapy in 40 antiretroviral naive, HIV-1 infected patients. Subject monitoring for the first 48 weeks is frequent, q 4 weeks. Primary objective of the trial is to determine the proportion of patients with HIV RNA <400 copies/mL at weeks 24 and 48.
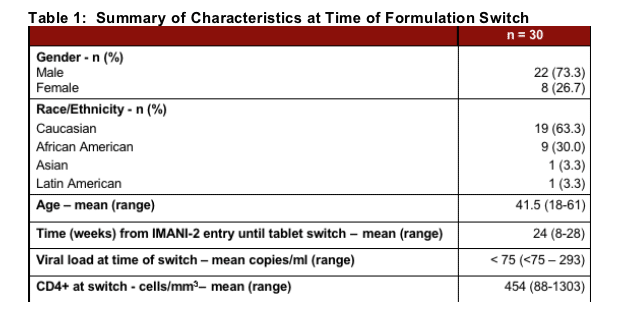
Thirty (30) of the IMANI-2 subjects had received at least 4 weeks of LPV/r SGC therapy at the time of enrollment for this substudy. All 30 were enrolled in the sub-study examining quality of life, tolerability and treatment preference prior to and following switch from LPV/r 400/100 mg bid soft gel capsule formulation to LPV/r 400/100 mg bid tablet formulation.
Key Entry Criteria
≥ 18 years of age
ARV naive
No current opportunistic infections or acute illness
No concurrent HCV interferon therapy
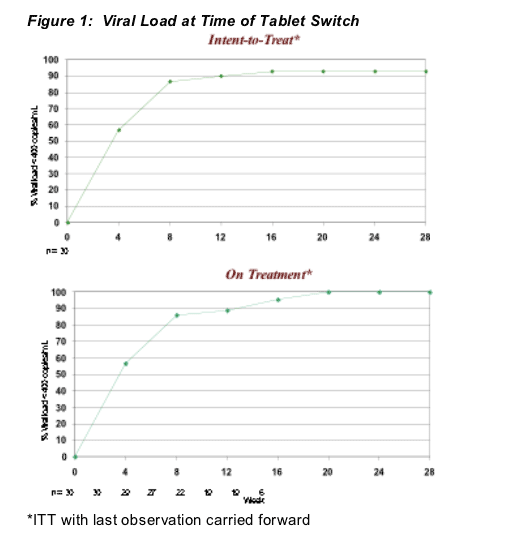
As a condition of the IRB, patients must have been responding to be retained on single agent therapy (responding defined as demonstrated one log-reduction in viral load by week 4 and virologic suppression < 400 copies/mL for those beyond week 16). All 30 patients who had reached at least 4 weeks at the time of this substudy, were responding. In addition, the IRB required that week 24 viral response be no more than five percentage points lower than LPV/r pivotal trial results to continue the study. (Figure 1)
STUDY DESIGN and ANALYSIS
Responding subjects were administered a series of questionnaires immediately prior to and 4 weeks following switch from soft gel capsule to tablet formulation. These include:
Medical Outcomes Study - HIV (MOS-HIV) - a validated instrument assessing quality of life consisting of 35 questions to assess physical, social, and emotional well being during the previous 4 weeks. Scores are standardized to a reference population with higher values representing better quality of life. Two summary scores are computed, including physical health summary score (PHSS) and mental health summary score (MHSS). [4]
Center for Epidemiologic Studies - Depression (CES-D) - a 20 item questionnaire assessing factors consistent with a depressive state during the previous week. [5] A score of 16 or higher indicates that the subject is experiencing symptoms of depression.
Modified Global Condition Improvement (GCI) - measures overall tolerability and HIV treatment preference.
Prevalence of diarrhea was captured via adverse event reporting prior to and following tablet switch.
Statistics were conducted using two-tailed t-tests for paired comparisons with 0.05 level of significance.
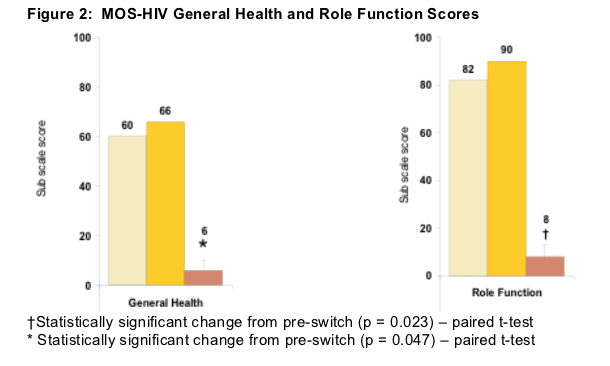
MOS-HIV scores are summarized in Figures 2 - 5 . Post-formulation switch switch, statistically significant increases in general health perception and role functioning were reported compared to baseline.
General health perception includes degree of illness, perception of health compared to others, degree of agreement that health is "excellent", and whether or not one has been feeling badly lately.
Role functioning includes whether or not one is able to work at a job or around the house, and the degree to which ability to do certain kinds of work has changed from baseline.
As you can see in the table below General Health Score was 60 before switch and 66 after switch. The 6 point improvement was statistically significant. The Role Function Score was 82 before switch and 90 after switch; the 8 point improvement was statistically significant.
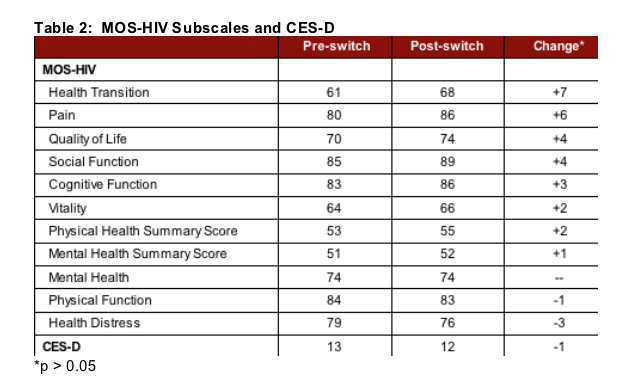
Modest improvements were also reported in overall quality of life, social functioning, health transition (e.g. perceived health compared to 4 weeks ago), and pain scores. Little or no changes were observed in overall physical functioning, reported mental health, vitality, or cognitive functioning, health distress, or the physical and mental health summary scores. There was also no significant difference in CES-D score from pre to post-switch. (Table 2)

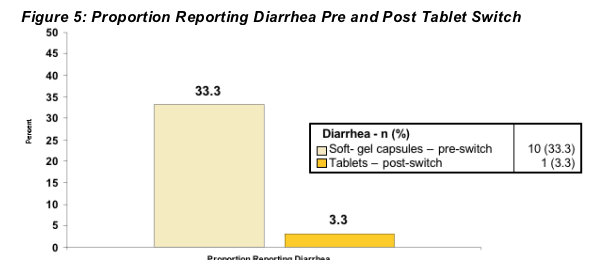
DIARRHEA
Ten patients (33.3%) reported diarrhea of at least Grade 2 at any time of enrollment until switch while receiving soft gel capsules. One patient* reported diarrhea (Grade 2) following switch to tablet formulation. (Figure 5) No patients discontinued trial for diarrhea or AE.
*Patient had diarrhea for 4 months prior to switch from soft-gel capsules to tablets and one report of diarrhea post switch that was possibly related to drug.
|
| |
|
 |
 |
|
|