 |
 |
 |
| |
Tenofovir Renal Affects in Mild Renal Impairment and Hypertension/Diabetes
|
| |
| |
Reported by Jules Levin
8th International Congress on
Drug Therapy in HIV Infection
November 12-16, 2006
Glasgow, UK
Note from Jules Levin: This report contains studies presented at Glasgow. The first is a poster from Gilead reporting median changes in renal function in patients with mild renal impairment or on hypertension and/or diabetes medications before starting tenofovir in comparison to taking d4T or AZT in 2 Gilead tenofovir studies (903 and 934). The study examines changes in renal function parameters through 96 weeks in creatinine and estimated glomerular filtration rate (GFR) by Cockcroft-Gault (CG) (mild renal impairment defined as 50-80 mL/min [0.83-1.33 mL/s], and MDRD. This analysis reports median changes in these values and reports no significant changes in creatinine or declines in median values in GFR in the tenofovir groups but patients in the other groups (AZT, d4T) experienced increases in GFR by CG and MDRD. There were several relevant posters and the abstracts are below.
"Renal Safety Profile of Tenofovir DF (TDF)-containing Compared to Non-TDF-containing Regimens in Antiretroviral-naive Patients with Mild Renal Impairment or Hypertension and/or Diabetes Mellitus"
S Staszewski,1 AL Pozniak,2 J Gallant,3 E DeJesus,4 SS Chen,5 J Enejosa,5 and A Cheng5
1University Hospital, J.W. Goethe-Universität, Frankfurt, Germany; 2Chelsea and Westminster Hosp, London, UK;
3Johns Hopkins Univ School of Med, Baltimore, MD, USA; 4Orlando Immunology Center, Orlando, FL, USA;
5Gilead Sciences, Inc., Foster City, CA, USA
INTRODUCTION
HIV+ patients with low CD4 cell counts or medical conditions such as hypertension or diabetes mellitus are at increased risk for renal impairment.
Due to decreased renal clearance of tenofovir, patients with mild renal impairment may be at increased risk for TDF-associated renal adverse events.
We investigated the renal safety profile of TDF in sub-populations from 2 randomized, controlled studies. (patients received either TDF/FTC, or TDF/3TC or d4T/3TC or AZT/3TC).
METHODS
We evaluated the renal parameters through 96 weeks in antiretroviral-naive
patients who initiated a TDF-containing vs a thymidine analog-containing
regimen (Control) through 96 weeks in Studies 903 and 934 and met the following criteria:
- Mild renal impairment at baseline
- Defined as an estimated glomerular filtration rate (GFR) by Cockcroft-Gault (CG) of 50-80 mL/min [0.83-1.33 mL/s]
- Taking concomitant antihypertensive and/or anti-diabetic medications
Main Inclusion Criteria
- HIV-1 infected patients naive to antiretroviral treatment
- 18-65 years of age
- Plasma HIV RNA > 5,000 copies/mL (Study 903); > 10,000 copies/mL (Study 934)
- No CD4+ cell count criteria
- No significant laboratory or clinical abnormalities
- Serum Creatinine < 1.5 mg/dL [< 133 _mol/L]
- Serum Phosphorus ≥2.2 mg/dL [≥0.71 mmol/L]
- Estimated GFR by Cockcroft-Gault ≥60 ml/min [≥1.0 mL/s] (Study 903);
≥50 ml/min [≥0.83 mL/s] (Study 934)
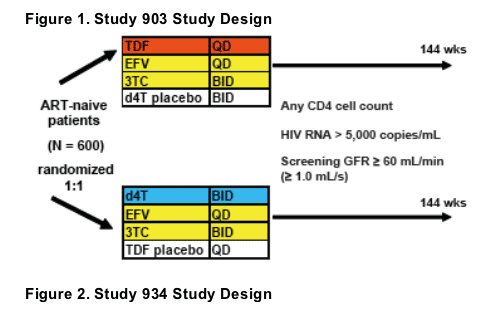
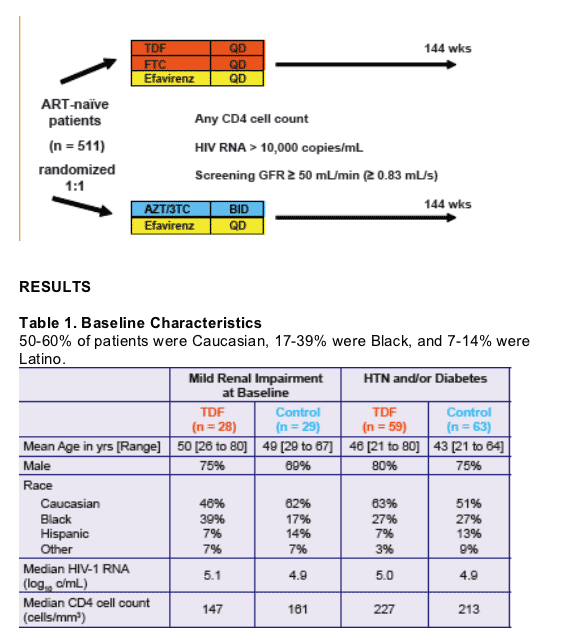
Table 2. Baseline Renal Parameters
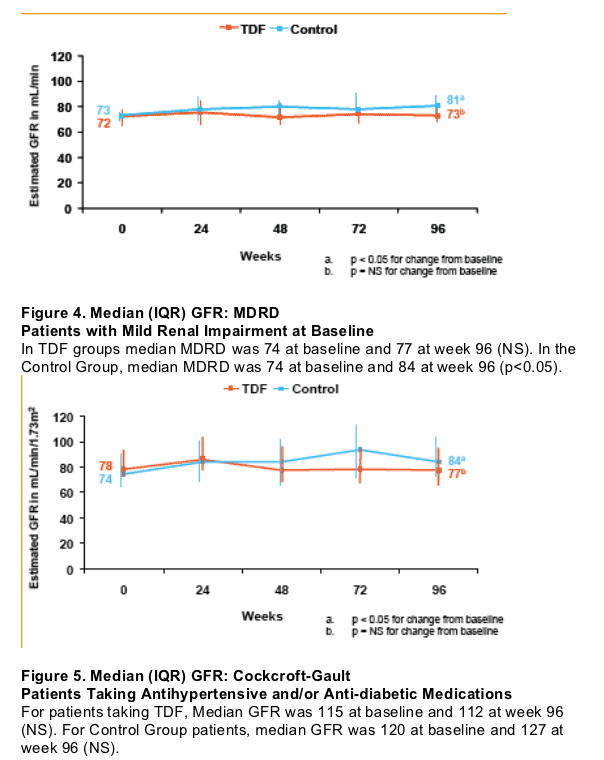
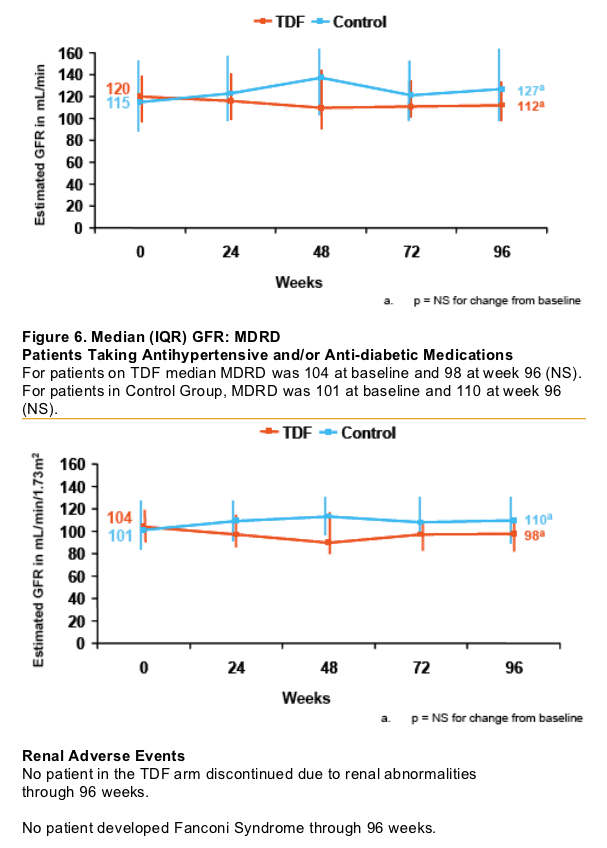
Baseline creatinine (mg/dL) was median of 1.1 for TDF & estimated GFR was 72 by CG (MDRD 78) in patient group with mild renal impairment, and 0.9 creatinine & est. GFR 120 by CG (MDRD 104) in patient group on TDF with hypertension and/or diabetes.
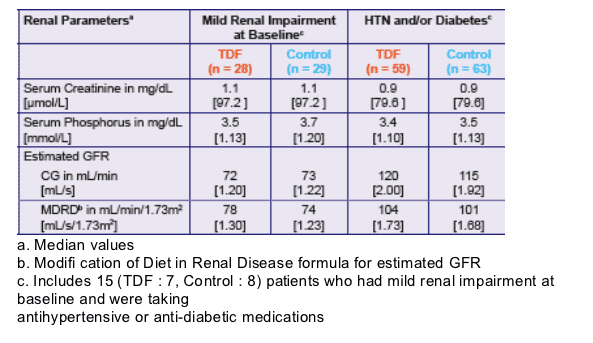
Table 3. Change from Baseline in Renal Parameters (median values)
Median creatinine did not change in either TDF groups. Serum phosphorous declined -0.30 from 3.5 mg/dL at baseline in TDF group with mild renal impairment, and -0.10 from 3.4 mg/dL at baseline. Median values for Estimated GFR did not change from baseline in either TDF group, by CG or MDRD. In the Mild Renal Impairment Control group median Estimated GFR increased by 8 from 73 at baseline (p<0.05) by CG and by 1 from 115 at baseline (NS) in the HTN and/or diabetes Control Group). Median MDRD also increased in Mild Renal Impairment Control Group by 11 from 74 at baseline (p<0.05). No change in MDRD in other Control Group.
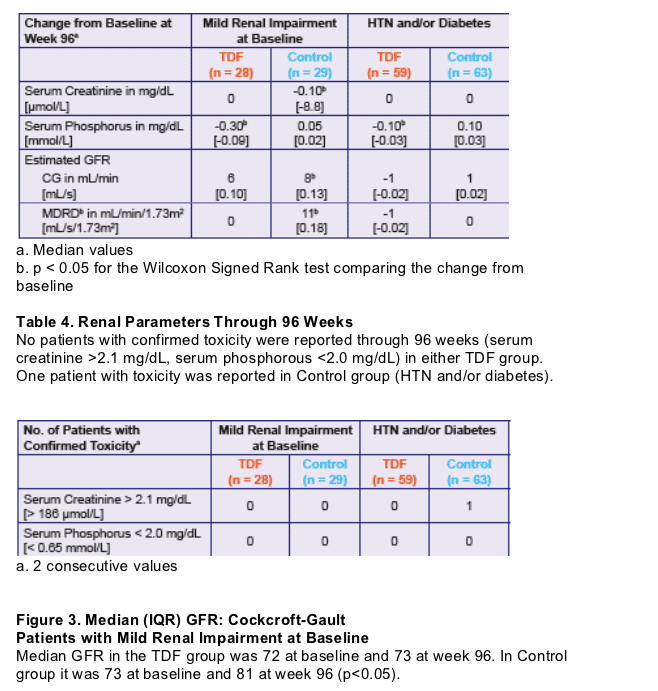
AUTHOR CONCLUSIONS
The renal safety profi le in patients with mild renal impairment, hypertension and/or diabetes mellitus receiving TDF-containing vs. thymidine analog-containing regimens through 96 weeks did not demonstrate an increased risk of renal dysfunction.
- No significant changes in estimated GFR by either CG or MDRD from baseline to Week 96 were observed in TDF arm
- No patient discontinued due to TDF-associated renal adverse events or laboratory abnormalities
- No patient developed Fanconi syndrome
No significant changes in estimated GFR by CG was observed through 96 weeks
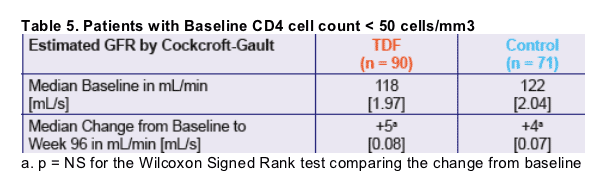
of antiretroviral therapy in patients with advanced immunosuppression (CD4 < 50) at baseline.
LATE BREAKER at GLASGOW
[PL13.2] Predictors of creatinine (Cr) increase and drug discontinuation in patients receiving tenofovir DF (TDF)
M Harris, R Joy, N Zalunardo, R Werb, B Yip, R Hogg, J Montaner AIDS Research Program, St. Pauls Hospital, Vancouver, BC, Canada; BC Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada; Division of Nephrology, University of BC, Vancouver, BC, Canada
Purpose of the Study TDF use in the EAP was associated with clinically significant Cr increases, seen earlier in patients with lower CD4. Since approval, TDF use is not restricted by previous ARV, CD4, renal function or concomitant medications. We examined factors associated with Cr increase and TDF discontinuations (d/c) using a database including ARV history and lab values.
Methods HIV+ adults starting TDF 01/01/03 to 31/05/05 and with 1 Cr within 6 mo of TDF start and 1 Cr while still on TDF were included. Endpoints were Cr 1.3x pre-TDF baseline (BL) or TDF d/c for any reason. Logistic regression was used to calculate unadjusted and adjusted odds ratios (OR). Event-free subjects were right censored at the last Cr test up to 31/10/05.
Summary of Results Analysis includes 1182 patients: 1000 male (85%), 188 ARV nave (16%), 265 with AIDS (22%), median age 42 yrs, previous ARV 34 mo, CD4 220/mm3, VL 24,500 c/mL, Cr 83 mol/L, GFR 92 mL/min/1.73m2. Concomitant ARV included ddI in 406 (34%) and boosted PIs in 967 (82%). Median time on TDF was 12.2 mo. Five % (62/826) developed Cr 1.3x BL and 20% (236/1182) d/ced TDF, including 66 (6%) who died. In multivariate analysis, factors associated with Cr 1.3x BL were concomitant ddI (OR 2.14; p=0.015), BL CD4 (OR 1.59/ 100 cell decrement; p<0.0001), and female gender (OR 2.33; p=0.029). Factors associated with TDF d/c were concomitant ddI (OR 2.21; p<0.0001), BL CD4 (OR 1.22/ 100 cell decrement; p<0.0001), and previous ARV exposure (OR 0.89/ 12 mo; p<0.0001).
Conclusions Among patients taking TDF, Cr elevation and TDF discontinuation are associated with concomitant use of ddI (but not boosted PIs), and with lower CD4, as previously shown.
POSTERS at GLASGOW
[P154] Nephrotoxicity in HIV patients receiving combination antiretroviral therapy including tenofovir: results from the SCOLTA Project
Giordano Madeddu, Elena Ricci, Giuseppe De Socio, Silvia Carradori, Carmela Grosso, Patrizia Marconi, Giovanni Penco, Elena Rosella, Sebastiano Miccolis, Sara Melzi, Simona Landonio, Carla Lovigu, Paolo Bonfanti, Tiziana Quirino. C.I.S.A.I. Group, Milan, Italy
Purpose of the Study To evaluate the prevalence and incidence of nephrotoxicity in patients (pts) enrolled in the SCOLTA Project tenofovir (TFV) cohort and to identify possible risk factors.
Methods The SCOLTA Project is a prospective, observational, multicenter study involving 25 infectious diseases departments in Italy created to assess the incidence of serious adverse events in pts treated with new antiretroviral drugs.
Summary of Results Data including grade II-IV creatinine elevations according to ACTG scale were available in 354 pts, 237 (67%) males, enrolled in the TFV cohort. Mean age was 40.1 ± 7.6 years, mean CD4 cell count 363 ± 259 cell/_L and mean HIV RNA 3.6 ± 1.4 log10 cp/ml; 179 (50.4%) pts were HCV Ab+ and 22 (6.2%) HBsAg+. During a mean follow up of 19.5 ± 11.5 months creatinine elevations were reported in 9/354 (2.5%) pts, all males. The overall incidence was 1.6 (95% CI 1.5-1.7) per 100 person-years (p-y) and 0.5 (95% CI 0.4-0.6) per 100 p-y for grade III. No grade IV event was reported. Mean duration of TFV therapy at the event was 9.5 ± 5 months. Pts with nephrotoxicity were older and more frequently male, HCV infected, in CDC stage C and their CD4 cell count was significantly lower than those without nephrotoxicity. Furthermore, 2 pts had chronic kidney disease at baseline. No significant difference was found between TFV co-administered antiretroviral drugs.
Conclusions Both prevalence and incidence of nephrotoxicity were low in pts receiving TFV in a non-selected clinical setting. Renal injury in pts receiving TFV seems associated with the presence of co-morbidities and with advanced HIV infection.
[P156] Renal function in patients taking tenofovir and non-tenofovir based antiretroviral regimens
M M Rosenvinge, B A Chelliah, J Goldblatt, S T Sadiq, M R Pakianathan. Genito-Urinary Medicine, St George's Hospital, London, UK; Statistics, Lancaster University, Lancaster, UK; St George's, University of London, London, UK
Purpose of the Study Renal toxicity, rarely observed in clinical trials, may be more frequently observed in clinical practice outside of trial entry criteria and in Black African patients.
Methods A retrospective, cohort, case-controlled study. Patients who started a tenofovir (n=100) or non-tenofovir containing regimen (n=127) between 1/1/04-1/6/05 were compared. Demographics, CDC stage, baseline viral load and CD4 count, pre-existing medical conditions, previous exposure to HAART and reason for starting treatment were collected. Weight, serum creatinine and phosphate was recorded at 0, 3, 6 and 12 months post HAART initiation. The creatinine clearance (CrCl) was calculated using the Cockcroft -Gault equation.
Summary of Results Preliminary results of 61 tenofovir cases (72% Black African) and 73 controls (59% Black African) indicated that the groups were comparable in age, gender and ethnicity. Tenofovir cases were more likely to be at CDC stage C and have had prior HAART (p<0.002 for both). Mean creatinine at baseline and 12 months for cases was 73_mol/l and 80_mol/l, respectively and for controls was 87_mol/l and 83_mol/l, respectively (NS). Mean CrCl at baseline and 12 months for cases was 112mls/min and 110mls/min, respectively and for controls was 120mls/min and 130mls/min, respectively (NS). At 12 months there was a mean increase in CrCl in the control arm of 10.2mls/min and a mean decrease in the tenofovir arm of 1.8mls/min (p=0.04).
Conclusions There was no significant increase in renal toxicity for patients taking tenofovir, although those on a non-tenofovir based regimen showed a greater improvement in CrCl. The clinical signficance of this can only be ascertained by longer term follow up.
[P155] Changes in creatinine clearance over time in a patient cohort on tenofovir as part of combined antiretroviral therapy
Imali Fernando, Dan Clutterbuck. Genito Urinary Medicine, New Royal Infirmary of Edinburgh, Edinburgh, Lothian, UK
Purpose of the Study To retrospectively overview changes over time in creatinine clearance in a patient cohort following initiation of Tenofovir DF (TDF) as part of combined therapy.
Methods This was a retrospective study of all patients (82) at the Edinburgh Genito Urinary Medicine clinic who received TDF as part of combination therapy for a period exceeding 6 months. Creatinine clearance values were calculated (using Modified Diet in Renal Disease equation) preceding the initiation of TDF and then, (while on the drug) at intervals of 1 month, 3 months, 6 months, 1 year, and annually thereafter.
Demographic data (age, race and sex) were collected. Note was also made of CD4 count and viral load at initiation of TDF, time from HIV diagnosis, CDC code, previous antiretroviral experience, previous recreational drug usage, hepatitis B and C co-infection, other significant comorbidities (e.g. - previous renal impairment, hypertension and diabetes) and concurrent nephrotoxic drug use.
Summary of Results Overall, there was no statistically significant change in creatinine clearance in this cohort to be noted over time (P>0.05).
Conclusions Previous studies showed a link between significant nephrotoxic events in this patient population and variables of baseline renal insufficiency, diabetes and advanced immunosuppression. It may be queried therefore whether the relative scarcity of these predisposing factors in the Edinburgh cohort helped prevent any significant renal events1, 2.
1. Becker S et al. Beyond Serum Creatinine: Identification of Renal Insufficiency Using Glomerular Filtration: Implications for Clinical Research and Care. Poster 819, 12th CROI 2005.
2. Gallant JE et al. Decline in Renal Function Associated with Tenofovir DF (TDF) Compared to Nucleoside Reverse Transcriptase Inhibitor (NRTI) Treatment. Poster N-138, 12th CROI 2005.
|
| |
|
 |
 |
|
|