 |
 |
 |
| |
Significant improvements in self-reported gastrointestinal tolerability, quality of life, patient satisfaction, and adherence with lopinavir/ritonavir after switching from BID soft-gel capsule (SGC) to BID Tablets
|
| |
| |
S Schrader1, SK Chuck2, LW Rahn2, KG Emrich2, PS Parekh21The Schrader Clinic, Houston, TX, USA,2Abbott Laboratories, Abbott Park, IL, USA
AUTHOR CONCLUSIONS
In this US survey of 332 HIV-infected patients, significant improvements were reported by patients switching from LPV/r SGC dosed BID to Tablets dosed BID:
-- 82% of respondents reported no or improved diarrhea
-- Additional 12% of respondents reported no or rare bloating, pain, gas in stomach
-- Significant improvements in satisfaction, as well as overall tolerability, for ∼20% of respondents
-- Adherence improved from 91% to 95%, with additional 7% of respondents reporting no missed doses
The LPV/r Tablet benefit most frequently cited by respondents were:
-- Don't have to refrigerate (67%),
-- fewer pills (61%),
-- don't have to take with food (41%)
These results suggest that LPV/r Tablets dosed BID provides multiple benefits to HIV patients relative to SGC. Additional study to further define thetolerability profile of LPV/r Tablets is warranted.
STUDY OBJECTIVES
To analyze ethnic differences in the following parameters based on patient self-report during the conversion of LPV/r SGC to Tablet formulations dosed 400/100mg twice a day
-- Patient preference between LPV/r SGC and Tablet formulations
-- Benefits
-- Adherence
-- Satisfaction-Missed doses, fewer pills
--Tolerability-Reasons for missed doses
-- Overall, frequency & severity of select side effects
-- Diarrhea & antidiarrheal therapy use
METHODS- SURVEY DESIGN
-- Self-reported, anonymous, multiple-choice survey in English & Spanish
-- Addresses satisfaction, overall tolerability, adverse effects, adherence, perceived benefits, formulation preference, and quality of life
-- SGC and Tablets surveys had identical questions with 4 additional comparative questions (SGC vs. Tablets) in the Tablets survey
-- Respondents were asked to think back over the last 4 weeks and indicate in a typical week the frequency & severity of side effects
-- Adherence was reported by respondents based on the last week of dosing
-- Questions written at grade 6 level
METHODS- SURVEY DISTRIBUTION
-- 52 out of 65 US physicians contacted distributed surveys to patients; a small payment to physicians were made for efforts related to distribution and handling of surveys with a maximum of 25 patients per site allowed; patients received no compensation
-- Physicians provided surveys to the patients while on LPV/r SGC and LPV/r Tablets dosed at 400/100mg BID after a minimum of 4 weeks on each formulation.
-- Patients completed the surveys in waiting area at their routine scheduled visits.
-- Patient privacy was maintained by having patients seal completed surveys into envelopes prior to providing survey to clinic staff for mailing to research company managing the project.
-- October 2005 through May 2006
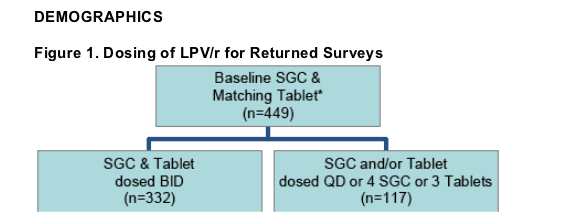
332 matched Tablet surveys with LPV/r SGC and Tablets dosed BID were returned from 52 physicians in 20 states & Washington, DC.
Respondents were mostly males (85%) with diverse ethnicity.
87% of respondents were >35 years old.
Duration of antiretroviral therapy:
- 59% at least 5 years
- 31% 1 to 5 years
- 10% < 1 year
Duration of LPV/r therapy:
- 82% of LPV/r SGC experience was > 1 year
- 89% of LPV/r Tablet experience was < 3 mos
Blacks: 37%
Whites: 40%
Hispanics: 20%
Other: 3%
RESULTS
Significantly more respondents indicated they were "extremely"or "very"satisfied after switching to LPV/r Tablets (80% vs. 60% on SGC, p<0.05)
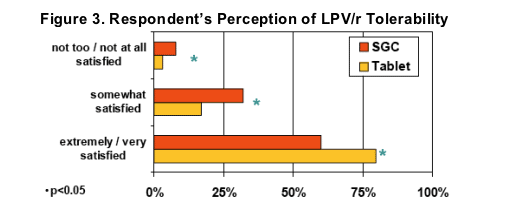
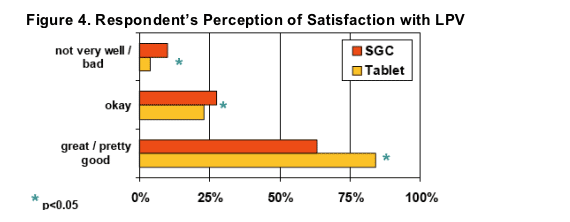
Significantly more respondents indicated "great"or "pretty good" tolerability (i.e., no side effects or not bad side effects) after switching to LPV/r Tablets (84% vs. 63% on SGC, p<0.05)
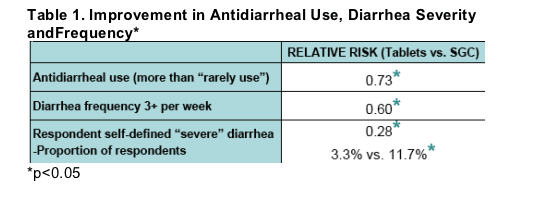
Respondents had significant improvements (p<0.05) in diarrhea frequency and severity after switching from LPV/r SGC to Tablets
82% reported no diarrhea or improvement.
An additional 21% more respondents indicated no or rare diarrhea(p<0.05).
Significantly fewer respondents reported bloating, gas, or pain in stomach, and those who did had diminished frequency on LPV/r Tablets
An additional 12% more respondents indicated no or rare occurrence (p<0.05).
Only 5% reported "severe"episodes (vs. 8% for SCG, p<0.10).
Self-reported adherence based on missed dosessignificantly improved after switching to LPV/r Tablets (95% vs. 91% for SGC, p<0.05)
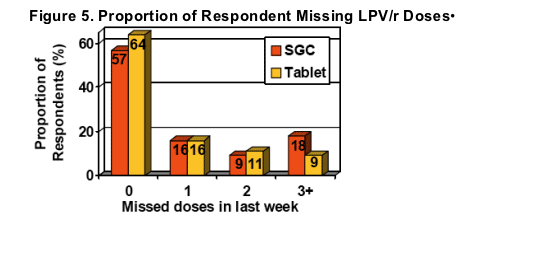
An additional 7% of respondents reported no missed doses with LPV/r Tablets.
Significant improvements were observed in 3 reasons for non-adherence after switching to LPV/r Tablets; avoiding side effects, running out, and not having food with them to take with the LPV/r dose
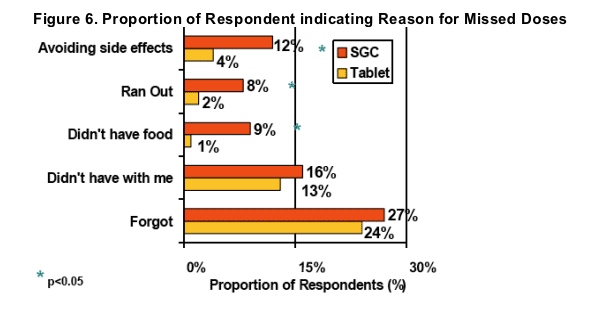
LPV/r Tablet benefits cited by respondents related to refrigeration, pill count, and food requirement
Respondents cited the following as benefits they "liked" -
-- Don't have to refrigerate (67%)
-- Fewer pills (61%)
-- Don't have to take with food (41%)
41% of respondents cited the lack of dietary restriction as a benefit. This may be explained by the similar level of non-adherence to LPV/r SGC's food requirement.
44% of respondents indicated at least 1 dose of LPV/r SGC was taken without food in the last week.
An average of 16% of LPV/r SGC doses were taken without food.
LPV/r Tablet was the preferred formulation over SGC by 88% (vs 3%) of respondents, with 79% of respondents stating that side effects were better with LPV/r Tab (vs 6%)
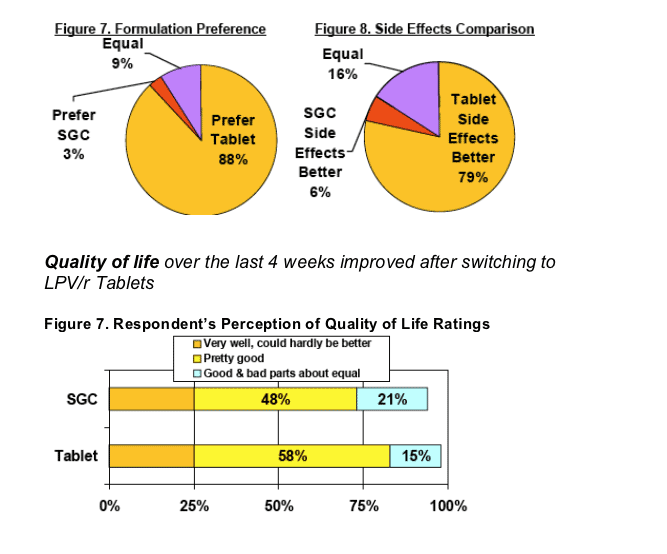
Significant improvements in quality of life ratings were observed with a shift from "good & bad parts about equal" to "pretty good"quality of life (p<0.05).
Quality of life improvements were noted by 73% and worsening by 2% of respondents when switched LPV/r Tablets.
|
| |
|
 |
 |
|
|