 |
 |
 |
| |
Three More Looks at First-Line Efavirenz vs Lopinavir
|
| |
| |
Glasgow Meeting: Part 3
8th International Congress on Drug Therapy in HIV Infection
November 12-16, 2006, Glasgow
Mark Mascolini
Following hard upon release of 96-week results from the AIDS Clinical Trials Group (ACTG) randomized trial comparing first-line efavirenz with lopinavir/ritonavir [1], three studies unveiled at the Glasgow meeting served up more data on the relative merits of these two potent antiretrovirals. All three studies found that efavirenz is as good as--or in some ways a little better than--lopinavir. But these studies have distinct limits. Only one is a randomized trial, and it's a small one with a soft primary endpoint. The other two reports rest on cohort studies.
Another randomized trial of efavirenz versus lopinavir
A 100-person Spanish-Italian study, the LAKE trial, found equivalent 24-week virologic and CD4 responses to efavirenz and lopinavir/ritonavir, both given in a first-line regimen with fixed-dose lamivudine (3TC)/abacavir (Kivexa) [2]. Early lipid trends looked better in the efavirenz group, but more people taking efavirenz had hypersensitivity reactions.
Patrician Echeverria (Hospital Universitari Germans Trias i Pujol, Barcelona) and colleagues from 18 other clinics randomized 55 previously untreated people to efavirenz and 45 to lopinavir/ritonavir. Pretreatment CD4 counts averaged 193 in the efavirenz group and 191 in the lopinavir group, but the researchers did not report pretreatment viral loads. Starting lab values were similar in the efavirenz and lopinavir groups--156 and 148 mg/dL for total cholesterol, 93 and 96 mg/dL for low-density lipoprotein (LDL) cholesterol, 49 and 44 mg/dL for high-density lipoprotein (HDL) cholesterol, 33 and 36 U/L for aspartate aminotransferase (AST), and 36 and 45 U/L for alanine aminotransferase (ALT).
After 24 weeks of treatment 54 people taking efavirenz (98%) and all 45 taking lopinavir/ritonavir had a viral load below 400 copies, the primary endpoint. Echeverria and coworkers did not report results with a 50-copy assay. CD4 gains were moderately greater with lopinavir than with efavirenz, but that difference lacked statistical significance. Transaminase changes did not differ between the two study arms. People randomized to efavirenz had a significant gain in "good" HDL cholesterol to 50 + 10 mg/dL (P = 0.021). Seven people taking efavirenz (12.7%) and 2 taking lopinavir (4.4%) had hypersensitivity reactions, a difference perhaps reflecting the compound hypersensitivity risk with a nonnucleoside and abacavir.
ACTG A5142, presented in August 2006 at the XVI International AIDS Conference, had two main endpoints--time to virologic failure (defined as either failure to push the viral load down 10-fold or rebound before week 32 OR failure to have a sub-200 load or a rebound after week 32) and time to regimen completion (defined as virologic failure or toxicity-related discontinuation of any drug in the regimen) [1]. After 96 weeks of follow-up efavirenz beat lopinavir/ritonavir on the first endpoint and matched the protease inhibitors (PIs) on the second endpoint.
Among ACTG trial participants who did endure virologic failure on efavirenz, preliminary analysis determined that a higher proportion ended up with double-class resistance than did people in whom lopinavir/ritonavir failed. Unlike the LAKE trial, ACTG A5142 found a significantly superior 96-week CD4 gain with lopinavir (285 versus 241, P = 0.01). But that statistical difference appeared to have no short-term clinical impact since disease progression rates did not differ between the lopinavir and efavirenz arm. Besides being four times longer than LAKE, the ACTG trial had five times as many people in the efavirenz-versus-lopinavir comparison.
Two cohort studies lean toward efavirenz
Efavirenz outperformed lopinavir/ritonavir in time to virologic failure and time to treatment failure in a retrospective cohort analysis involving 1159 treatment-naive people starting efavirenz and two nucleosides and 391 starting lopinavir and two nucleosides [3]. As in any cohort comparison, these results lack the vigor of a randomized trial because potential biases can never be entirely eliminated. Still, the Spanish SUSKA study does underpin the ACTG finding of faster time to virologic failure with lopinavir/ritonavir. In both SUSKA and ACTG 5142, the Kaletra Soft-Gel Capsules were used rather than the new tablets version of Kaletra.
People starting lopinavir in this cohort were older than those starting efavirenz (median 39.7 versus 37.8 years), they had a significantly lower median CD4 count (120 versus 187), and significantly more lopinavir takers had a pretreatment count under 100 (46.3% versus 31.5%) (P < 0.05 for the two CD4 comparisons). A larger proportion of people starting lopinavir had a pretreatment load above 100,000 copies (64.5% versus 57.6%), and a significantly higher proportion already had AIDS (35.8% versus 25.8%) (P < 0.05 for both comparisons). Similar proportions in each group had hepatitis B or C infection.
Through 144 weeks of follow-up, equivalent proportions in the lopinavir and efavirenz groups had a viral load under 400 copies or under 50 copies. And CD4 counts climbed proportionately in the two treatment groups. Time to virologic failure (not defined in the SUSKA poster) proved significantly faster with lopinavir (P < 0.05). The researchers defined treatment failure as virologic failure, or stopping efavirenz or lopinavir/ritonavir, or a new AIDS-defining illness, or death. By those criteria, time to treatment failure was significantly faster with lopinavir/ritonavir (P < 0.05).
In a multivariate analysis accounting for pretreatment differences between the two groups, treatment failure proved almost 30% more likely with lopinavir than with efavirenz (hazard ratio 1.297, 95% confidence interval 1.085 to 1.576, P = 0.0089). Analyses restricted to people beginning treatment with fewer than 100 CD4 cells, with more than 100,000 HIV RNA copies, with an AIDS diagnosis, or with HCV coinfection also favored efavirenz.
Grade 3 or 4 lab abnormalities were rare in this cohort, but rates tended to favor lopinavir over efavirenz. Higher percentages of efavirenz-treated people had any lab abnormality (6.29% versus 4.72%), hemoglobin below 7.9 g/dL (0.13% versus 0%), LDL cholesterol above 190 mg/dL (2.57% versus 1.57%), AST more than 5 times the upper limit of normal (4.11% versus 2.76%), and ALT more than 5 times the upper limit of normal (1.67% versus 0%). Higher proportions taking lopinavir (1.27% versus 0.39%) had triglycerides above 750 mg/dL.
A second Spanish cohort study involved people who began first-line efavirenz or lopinavir/ritonavir with a CD4 count under 100 from January 2002 through December 2004 [4]. Depending on the statistical analysis used, efavirenz proved virologically equivalent or superior to lopinavir. In this study as well, the older formulation of kaletra, the Soft-Gel Capsules were used rather than the new tablets.
The cohort embraced 1160 people from 93 hospitals, 665 starting efavirenz and 495 lopinavir/ritonavir. Median age measured 38 in the efavirenz group and 39 in the lopinavir group, equivalent proportions picked up HIV while injecting drugs (34%) and having sex (65%), and equivalent proportions had hepatitis B or C coinfection (40%). Median CD4 counts stood at 59.3 in the efavirenz group and 64.0 in the lopinavir group, and median viral loads registered 5.26 log and 5.30 log (about 182,000 and 199,500 copies).
The primary endpoint was time to treatment failure, defined as virologic failure, death, opportunistic infection, or treatment discontinuation. Statisticians also performed sensitivity analysis, defined in the table below the primary endpoint. Median time to treatment failure measured 175 weeks for efavirenz and 136 weeks for lopinavir/ritonavir, a difference that fell short of statistical significance (P = 0.11) (Table).
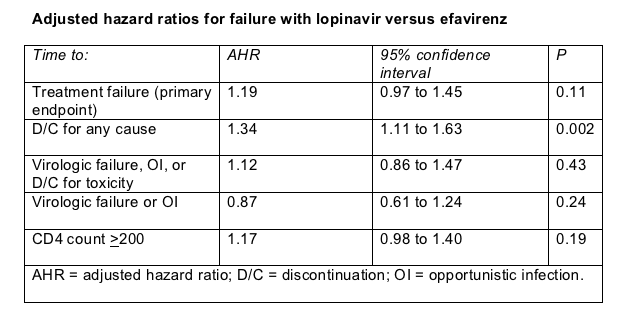
For two of three sensitivity analyses, starting with efavirenz or lopinavir/ritonavir also proved virologically equivalent (Table). But for the sensitivity analysis reckoning time to treatment discontinuation for any cause, efavirenz proved superior to the PIs (Table), partly because the efavirenz group had significantly fewer toxicity-related discontinuations than the lopinavir group (6.9% versus 11.1%, P = 0.012). Total discontinuations included regimen simplifications and stopping efavirenz because of pregnancy. ALT or AST elevations occurred in 13.2% taking efavirenz and 13.9% taking lopinavir. Among hepatitis virus-infected people, 5% taking efavirenz and 3.1% taking lopinavir had ALT or AST jumps. A significantly higher proportion taking lopinavir saw their triglycerides climb above 500 mg/dL (8.9% versus 4.1% (P = 0.001).
Figuring time to a CD4 count above 200 in people without virologic failure, the researchers plotted equivalent gains with efavirenz and lopinavir (Table).
These two cohorts and participants in the small randomized trial and in ACTG A5142 took the old gelatin-capsule lopinavir/ritonavir. That may have put them at a disadvantage because the new tablets appear to increase tolerability and adherence. For example, a 332-person US survey reported in Glasgow found that people switching from twice-daily capsules to twice-daily tablets had less diarrhea and other gastrointestinal upsets--and improved adherence--after the switch [click on the link at reference 5 for details].
For the record, two published cohort studies compared up-front efavirenz with lopinavir/ritonavir. One favored efavirenz, while the other rated the contest a draw. Italian MASTER Cohort researchers figured sub-50-copy rates for 348 antiretroviral-naive people starting efavirenz and 124 starting lopinavir/ritonavir [6]. Intent-to-treat analysis determined that after 24 weeks of therapy people starting the PIs had about half the chance of attaining viral suppression as people starting efavirenz (adjusted odds ratio 0.54, 95% CI 0.33 to 0.89, P = 0.016). Lopinavir users had a 60% lower chance of sub-50 suppression after 48 weeks (adjusted odds ratio 0.40, 95% CI 0.33 to 0.89, P = 0.002). But as in ACTG A5142 [1], CD4 gains proved significantly superior with lopinavir/ritonavir than with efavirenz in the MASTER cohort.
UK CHIC cohort investigators compared rebound rates by drug in people who reached a viral load below 50 copies with their first potent regimen then had two or more consecutive loads above 500 copies [7]. Among 3565 previously untreated people, those starting efavirenz had a lower rebound rate than those starting lopinavir/ritonavir, soft-gel saquinavir, indinavir, indinavir/ritonavir, saquinavir/ritonavir, nelfinavir, nevirapine, or abacavir. The rebound risk was 23% higher with lopinavir than with efavirenz, but that higher risk lacked statistical significance (rate ratio 1.23, 95% CI 0.58 to 2.59). Efavirenz was significantly more durable than indinavir/ritonavir, nelfinavir, nevirapine, or abacavir in this British cohort.
Mark Mascolini writes about HIV infection (markmascolini@earthlink.net).
References
1. Riddler SA, Haubrich R, DiRienzo G, et al. A prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV infection--ACTG 5142. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THLB0204.
2. Echeverria P, Carosi G, Galves J, et al. Similar antiviral efficacy and tolerability between efavirenz and lopinavir/ritonavir, administered with abacavir/lamivudine (Kivexa), in naive patients: a multicentre, randomized study. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract P7.
3. Domingo P, Teira R, Suarez-Lozano I, et al. Efavirenz vs. lopinavir/ritonavir -based combined antiretroviral therapy in antiretroviral-naive HIV-infected patients from the Spanish VACH cohort: the SUSKA study. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract P4.
4. Pulido F, Arribas J, Moreno S, et al. Similar virologic and immunologic response to efavirenz or lopinavir/ritonavir-based HAART in a large cohort of antiretroviral-naive patients with advanced HIV infection. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract 9.
5. Schrader S, Chuck SK, Rahn LW, et al. Significant improvements in self-reported gastrointestinal tolerability, quality of life, patient satisfaction, and adherence with lopinavir/ritonavir after switching from BID soft-gel capsule to BID tablets. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract 103. Go to http://www.natap.org/2006/Glasgow/Glasgow_36.htm for details.
6. Torti C, Maggiolo F, Patroni A, et al. Exploratory analysis for the evaluation of lopinavir/ritonavir-versus efavirenz-based HAART regimens in antiretroviral-naive HIV-positive patients: results from the Italian MASTER Cohort. J Antimicrob Chemother. 2005;56:190-195.
7. Smith CJ, Phillips AN, Hill T, et al. The rate of viral rebound after attainment of an HIV load <50 copies/mL according to specific antiretroviral drugs in use: results from a multicenter cohort study. J Infect Dis. 2005;192:1387-1397.
|
| |
|
 |
 |
|
|