 |
 |
 |
| |
Pharmacokinetic interaction between darunavir (TMC114), a new protease inhibitor, and methadone
|
| |
| |
Reported by Jules Levin
8th International Congress on Drug Therapy in HIV Infection, Glasgow, UK, 12-16 November 2006.
Sekar V,1 El Malt M,2 De Paepe E,2 Stevens T,2 De Pauw M,2 Vangeneugden T,2 Lefebvre E,1 Van den Brink W,3 Hoetelmans R2
1Tibotec Inc., Yardley, PA, USA; 2Tibotec BVBA, Mechelen, Belgium; 3University of Amsterdam, Amsterdam, The Netherlands
AUTHOR CONCLUSIONS
When DRV/r 600/100mg bid was added to stable individualised methadone
therapy, mean Cmin, Cmax, and AUC24h values of the biologically active methadone R-isomer were decreased by 15%, 24%, and 16%, respectively, compared with treatment with methadone alone. Mean Cmin, Cmax, and AUC24h values of the methadone S-isomer were also decreased by 40%, 44%, and 36%, respectively, after the addition of DRV/r to methadone treatment.
Symptoms potentially associated with opiate withdrawal were experienced by
four volunteers (including one volunteer for whom drug withdrawal syndrome
was reported as a separate AE) during the combined treatment phase.
Based on these findings, no a priori adjustment of methadone dosage is required
when methadone and DRV/r are co-administered. However, clinical monitoring is
recommended as maintenance therapy may need to be altered in some patients.
Methadone is a synthetic narcotic analgesic administered as a combination of the
biologically inactive S- and the biologically active R-isomers.6 A large number of HIV-infected patients with a previous history of intravenous drug use receive methadone as maintenance therapy, and interactions with ARVs have been observed.7 These interactions can precipitate withdrawal symptoms and warrant close monitoring.
DRV, RTV and methadone are substrates of the CYP3A4 enzyme. Additional metabolism of methadone occurs via CYP2B6, which is induced by RTV.8
The primary objective of this trial was to investigate the potential effect of DRV/r at steady-state on the steady-state pharmacokinetic (PK) parameters of methadone. The potential pharmacodynamic effects of methadone withdrawal in the presence of DRV/r and short-term safety and tolerability of the combination were also evaluated. The secondary objective was to investigate the effect of methadone on the pharmacokinetics of DRV (historical controls).
Study design
This study (TMC114-C127) was a Phase I, open label, add-on trial in 16 HIV-negative opioid-dependent volunteers receiving once-daily methadone maintenance therapy at a stable individualised dose of 55-200mg (SymoronŽ tablets, Yamanouchi Pharmaceuticals). Volunteers received DRV/r 600/100mg bid for 7 days in addition to their current methadone therapy, to assess the effect of DRV/r on the PK of the R- and S-isomers of methadone. A pharmacodynamic assessment of the symptoms of methadone withdrawal and an evaluation of short-term safety and tolerability were also conducted.
Major exclusion criteria included a positive HIV-1 or HIV-2 test at screening, female (unless postmenopausal for more than 2 years, post-hysterectomy or post-tubal ligation), and evidence of current use of barbiturates, amphetamine, recreational drugs or opioids (with the exception of methadone and cannabinoids).
The study protocol was reviewed and approved by the appropriate institutional ethics committee, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all volunteers.
Pharmacokinetic analysis
PK profiles of both methadone isomers were determined on Days -1 and 7 up to 24 hours post-dose. PK profiles of DRV and RTV were determined on Day 7 up to 12 hours post-dose.
Plasma concentrations of methadone, DRV and RTV were determined using validated liquid chromatography mass spectrometry/mass spectrometry methods. Bioanalysis of methadone was performed by ABL BV, Assen, The Netherlands; bioanalysis of DRV and RTV was performed by J&J PRD, Beerse, Belgium.
Predose plasma concentrations of methadone isomers on Days -2, -1, 5, 6 and 7 were compared graphically to assess the achievement of steady-state conditions of methadone on Days -1 and 7, respectively. For DRV and RTV predose concentrations in the morning of Days 5, 6 and 7 were similarly compared to examine steady-state conditions of DRV and RTV on Day 7.
Statistical analysis for methadone isomers compared pharmacokinetics on Day 7 (test: methadone [R- and S- isomers] + DRV/r) with those on Day -1 (reference: methadone alone). The primary PK parameters were minimum plasma concentration (Cmin), maximum plasma concentration (Cmax) and area under the plasma concentration-time
curve (AUC24h) on the logarithmic scale. All observations for test and reference, paired
and unpaired, were included in the statistical analysis.
The least square means (LSM) of the primary PK parameters for each treatment group were estimated with a linear mixed effects model, controlling for treatment as fixed effect and individual as a random effect. The 90% confidence intervals (CIs) around the
LSM ratios were also calculated.
Results
A total of 22 volunteers were screened: six were not treated due to not fulfilling all
inclusion/exclusion criteria, and 16 were treated and completed the trial. Overall,
15 (94%) of the volunteers were male, with a median age of 45 years (range
30-55 years). All were HIV-negative.
Pharmacokinetics of methadone isomers
Predose plasma concentrations for each methadone isomer were comparable between Days -2, -1, 5, 6 and 7, showing steady-state conditions were achieved prior to PK assessments on Day -1 and Day 7.
The mean plasma concentration profiles of the methadone isomers revealed that
co-administration of methadone and DRV/r resulted in lower systemic exposure to both isomers compared with administration of methadone alone, with a greater
decrease in S-isomer exposure than R-isomer exposure (Figure 1).
The mean values of the PK parameters at steady-state for both the R- and S-isomers of methadone were lower after intake of methadone in the presence of DRV/r compared with administration of methadone alone (Tables 1 and 2). After adding DRV/r, the median time to reach the maximum plasma concentration (tmax) was similar for R-methadone (Table 1), but median tmax for S-methadone increased from 3.0 to 4.0 hours (Table 2).
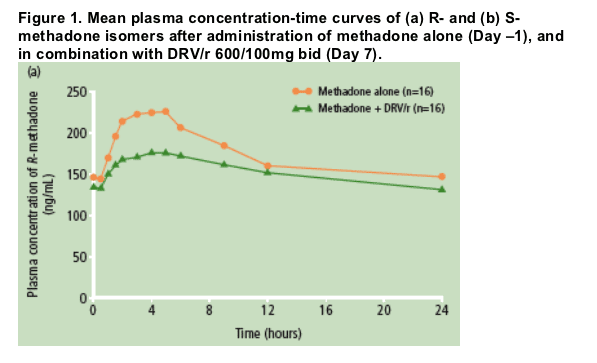
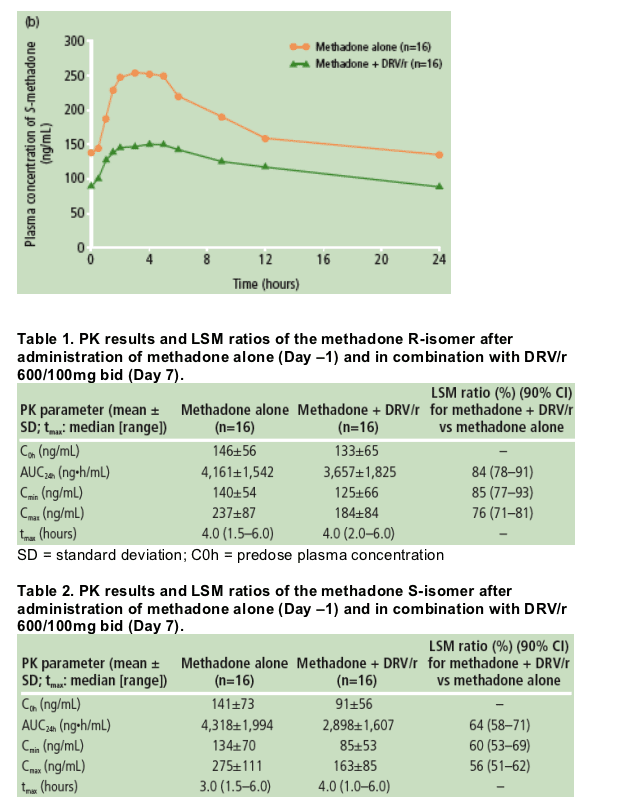
The differences in individual maintenance doses of methadone may explain the inter-individual variability of the methadone concentration-time profiles and PK parameters. Inter-individual variability for C0h, Cmin, Cmax, and AUC24h of R-methadone was
- 39%, 38%, 37% and 37%, respectively, for treatment with methadone alone
- 49%, 53%, 46% and 50%, respectively, in the presence of DRV/r.
Based on the LSM ratios, mean values of R-methadone Cmin, Cmax, and AUC24h were decreased by 15%, 24%, and 16%, respectively, when DRV/r was co-administered with methadone.
Variability in C0h, Cmin, Cmax, and AUC24h of S-methadone was similarly varied
- 52%, 53%, 40% and 46%, respectively, for treatment with methadone alone
- 61%, 63%, 52%, and 55%, respectively, in the presence of DRV/r.
Based on analysis of the LSM ratios, mean Cmin, Cmax, and AUC24h values for
S-methadone were decreased by 40%, 44%, and 36%, respectively, when DRV/r
was co-administered with methadone.
Changes in exposure to the methadone R-isomer and precipitation of
drug-withdrawal symptoms
Correlation analyses between changes in the AUC24h of R-methadone and symptoms potentially associated with opiate withdrawal as reported by the trial investigator (including sweating, shaking and insomnia, for which oxazepam was administered as a concomitant medication) showed that the four volunteers who experienced these symptoms (including one volunteer for whom 'drug withdrawal syndrome' was also reported as a grade 2 adverse event [AE]) had a decrease in methadone exposure; however, of the 12 volunteers who did not exhibit these symptoms, four had similar decreases in R-methadone exposure.
Pharmacodynamic assessment of methadone withdrawal did not reveal any clinically relevant changes in median values for the short opiate withdrawal scale, desires for drugs questionnaire scores and median pupil dilation versus reference.
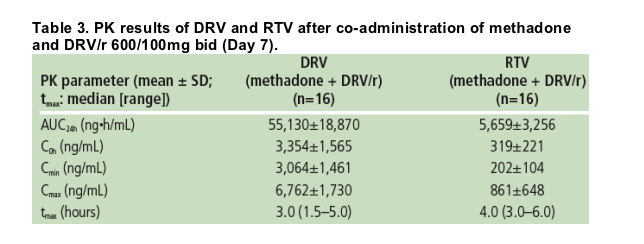
Pharmacokinetics of DRV and RTV
The values for the main PK parameters of DRV and RTV are shown in Table 3.
Inter-individual variability of C0h, Cmin, Cmax and AUC12h values for DRV was 47%, 48%, 26% and 34%, respectively. For RTV, these values were 69%, 51%, 75% and 58%, respectively.
Exposure to DRV following co-administration of DRV/r 600/100mg bid in the presence of methadone was in the range of DRV exposure observed in other studies.9,10
Safety
All 16 volunteers who received study medication completed the trial.
Fourteen (88%) volunteers reported at least one AE during the trial. Overall, the incidence of AEs was higher during treatment with DRV/r and methadone (88%) compared with methadone alone (31-38%) (Table 4).
No serious AEs or AEs leading to discontinuation were reported.
The majority of AEs were grade 1 in severity; no grade 3 or 4 AEs were observed. AEs observed in more than two volunteers during the combined treatment phase are shown in Table 4 for each phase of the trial.
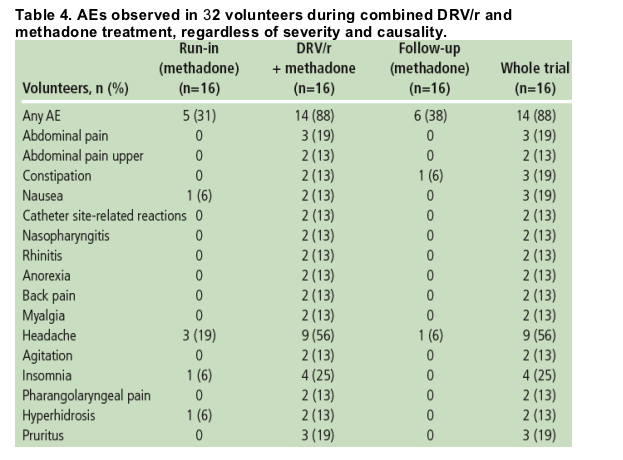
Headache was the most commonly reported AE during the trial, reported with a higher incidence during combined DRV/r and methadone treatment than during treatment with methadone alone (nine volunteers [56%] versus three [19%], respectively). All cases were considered grade 1 in severity, except for one grade 2 case during combined treatment. Insomnia and abdominal pain (upper) were also more commonly reported during combined DRV/r and methadone treatment than with methadone alone.
The AE of drug-withdrawal syndrome was reported as a grade 2 event in two (13%) volunteers, one during the run-in phase and one during DRV/r and methadone co-administration.
No clinically relevant changes over time in laboratory parameters were observed and no laboratory abnormalities were reported as AEs. All laboratory abnormalities were grade 1 or 2 in severity and observed in at most two volunteers during DRV/r and methadone co-administration, except for increases in total cholesterol, observed in five (31%) volunteers during combined treatment with DRV/r and methadone, compared with two (13%) during the run-in phase, and eight (50%) during follow-up.
No methadone dose adjustments were made during combined treatment with DRV/r and methadone for any volunteer.
References
1. Tibotec Inc. PREZISTATM (darunavir) Prescribing Information. June 2006 [cited 11 September 2006]. Available from:
http://www.tibotectherapeutics.com/pi.jsp.
2. Katlama C, et al. 3rd IAS Conference on HIV Pathogenesis and Treatment, Rio de Janeiro, Brazil, 24-27 July 2005. Abstract WeOaLB0.102.
3. Wilkin T, et al. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy,Washington DC, USA, 16-19 December 2005. Abstract 2860.
4. Lazzarin A, et al. 16th International AIDS Conference, Toronto, Canada, 13-18 August 2006. Abstract TUAB0104.
5. Sekar V, et al. 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO, USA, 5-9 February 2006. Abstract J-121.
6. Eap CB, et al. Drug Alcohol Depend 2000;61:47-54.
7. McCance-Katz EF, et al. Clin Infect Dis 2003;37:476-82.
8. Yamanouchi Pharmaceuticals. SymoronŽ (methadone hydrochloride) Prescribing Information February 2004.
9. Lefebvre E, et al. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 27-30 September 2006. Abstract A-0368.
10. Tibotec BVBA, Belgium. Data on file.
|
| |
|
 |
 |
|
|