 |
 |
 |
| |
Pharmacokinetics of TMC125 with atazanavir and atazanavir/ritonavir
|
| |
| |
Reported by Jules Levin
Eighth International Congress on Drug Therapy
in HIV Infection
Glasgow, UK, 12-16 November 2006
M Schöller-Gyüre,1 B Woodfall,1 T De Marez,2 G De Smedt,1 M Peeters,1 K Vandermeulen,1 R Hoetelmans1
1Tibotec BVBA, Mechelen, Belgium; 2Tibotec Inc., Yardley, USA
Background
TMC125 is an NNRTI with potent activity against wild-type HIV-1 and HIV-1
resistant to currently approved NNRTIs. TMC125 is eliminated via the hepatic
system. Atazanavir (ATV) may be given with low-dose ritonavir (ATV/r) and is an
inhibitor of several CYP450 enzymes. We evaluated the pharmacokinetics when
co-administering TMC125 (Phase II formulation) with ATV (400mg) and ATV/r
(300/100mg).
Methods
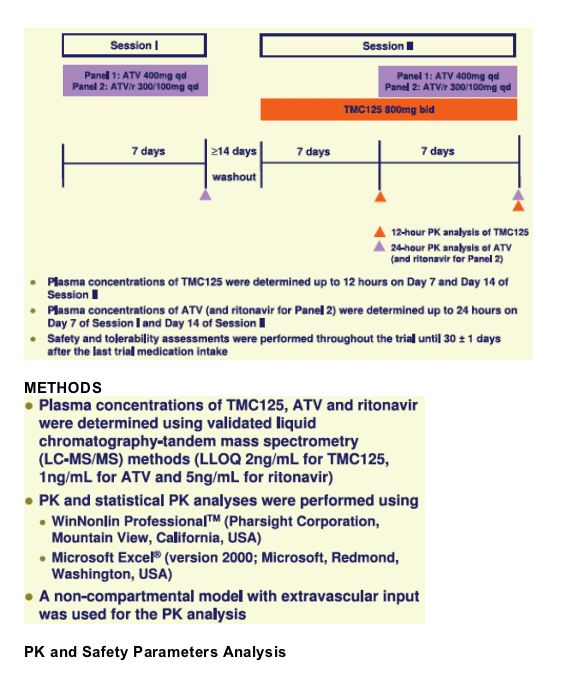
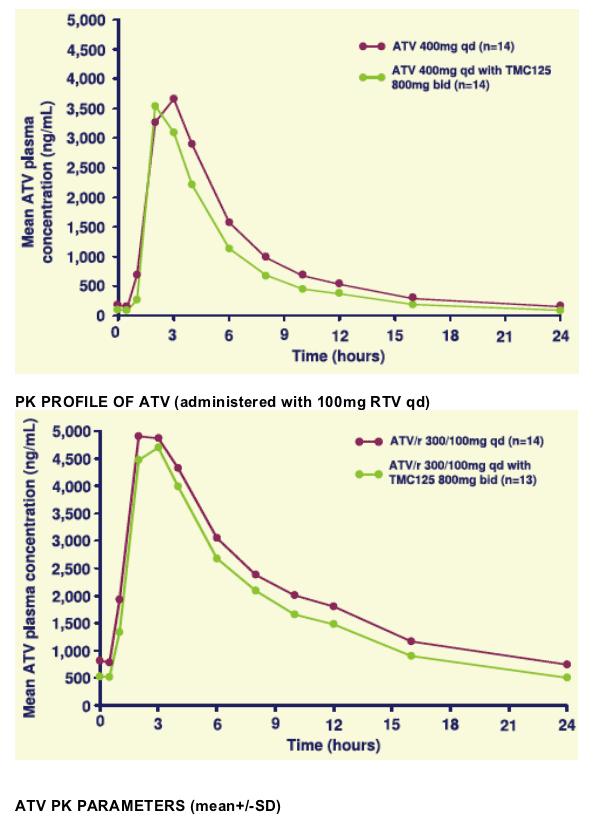
Volunteers in Panel 1 received ATV 400mg qd for 7 days (Session I) and TMC125 800mg bid for 14 days with ATV 400mg qd from Days 8 to 14 (Session II). Panel 2 received ATV/r 300/100mg instead of unboosted ATV in both sessions. Full pharmacokinetic (PK) profiles of TMC125, ATV and ritonavir were evaluated on Days 7 and 14. PK parameters were calculated by non-compartmental methods and analysed using a linear, mixed-effects model. Safety and tolerability were evaluated.
Results
Thirty two HIV-negative volunteers participated (30 male, median age 28 years).
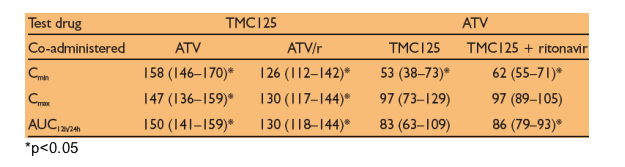
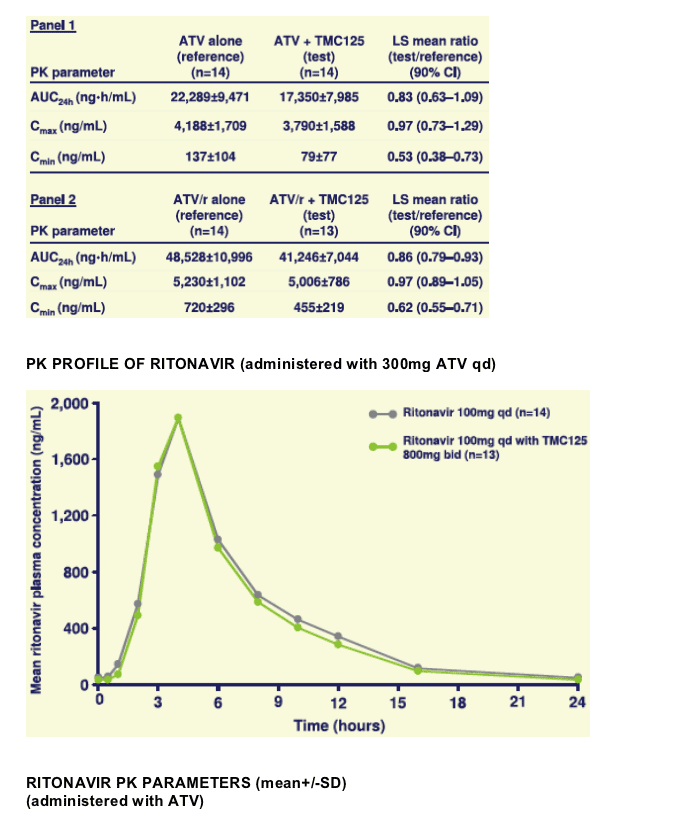
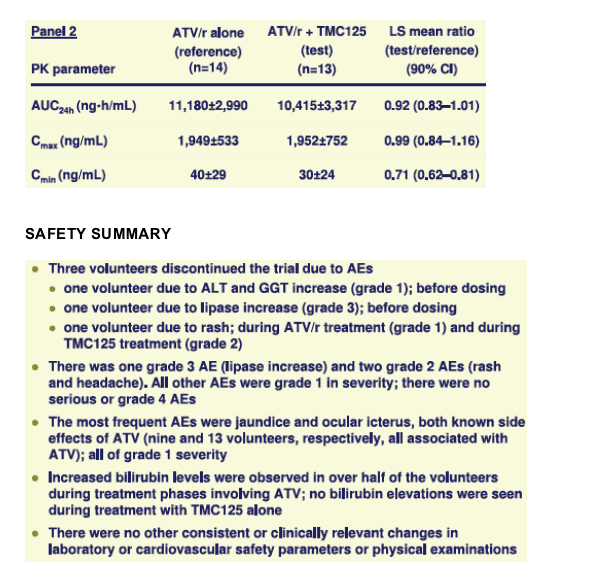
Least square (LS) means ratios (%) (90% CIs) for PK parameters obtained during combined administration versus treatment alone are given in the table below.
The most frequent adverse event (AE) was ocular icterus. Three volunteers
discontinued the trial due to AEs (two pre-dose laboratory abnormalities, one rash).
Conclusions
The increase of exposure to TMC125 when co-administered with either ATV or
ATV/r is considered not to be clinically relevant. However, due to the significant
decrease of Cmin of ATV when given with TMC125, this combination should only be used if combined with low-dose ritonavir.
AUTHOR CONCLUSIONS
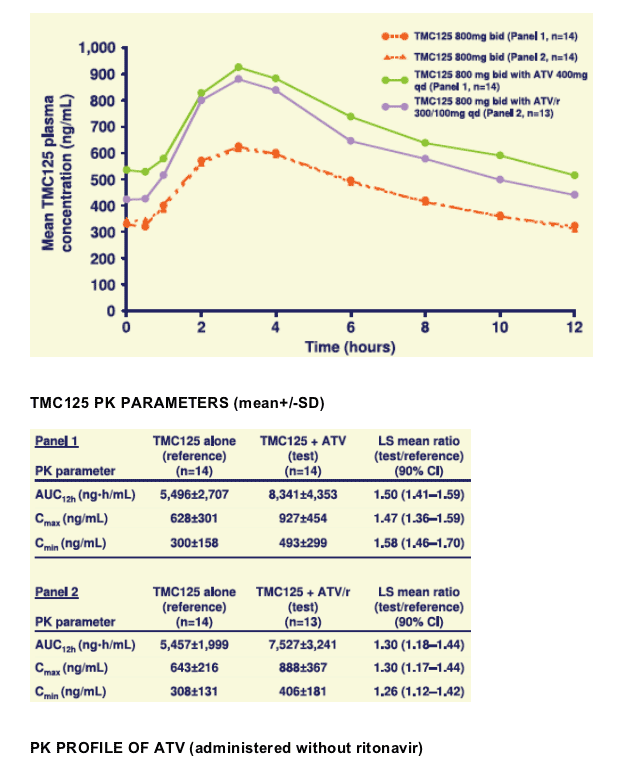
When co-administered with ATV or ATV/r, TMC125 exposure was increased by 50% and 30%, respectively. Similar increases were seen in Cmax and Cmin, probably due to inhibition of CYP450 enzymes and/or glucuronidation by ATV.
This increase of exposure is not believed to be clinically relevant given the safety profile of TMC125 and that no PK/safety relationship has so far been observed in clinical studies.
When combined with TMC125, the exposure to ATV was decreased by 14% and 17% when administered with and without low-dose ritonavir, respectively. This was possibly due to induction of CYP3A4 by TMC125. In the presence of TMC125, the minimum plasma concentration of ATV was decreased by 47% without low-dose ritonavir and by 38% with low-dose ritonavir.
Short-term co-administration of TMC125 with ATV with or without low-dose ritonavir in HIV-negative volunteers was generally safe and well tolerated. The nature of AEs reported during co-administration of TMC125 and ATV was consistent with the safety profiles of the individual drugs.
Due to the significant decrease in Cmin of ATV when given with TMC125, this combination should only be used if given with low-dose ritonavir, when ATV plasma concentrations are substantially higher.

References
1. Vingerhoets J, et al. J Virol 2005;79:12773-82.
2. Cohen C, et al. 16th International AIDS Conference 2006 (Poster TUPE0061).
3. Reyataz Prescribing Information, Bristol Myers Squibb Company (Princeton, NJ, USA). June 2006.
|
| |
|
 |
 |
|
|