 |
 |
 |
| |
Boosted PIs and Other First-Line Strategies
|
| |
| |
Glasgow Meeting: Part 4
8th International Congress on Drug Therapy in HIV Infection
November 12-16, 2006, Glasgow
Mark Mascolini
Four trials presented at the Glasgow meeting offered insights into first-line fosamprenavir/ritonavir, and three of them appraised 100 mg of ritonavir once daily as the boosting dose. Other investigators sized up the merits of first-line boosted tipranavir or saquinavir, fixed-dose abacavir/lamivudine (3TC) plus atazanavir/ritonavir once daily, and an induction-maintenance scheme that fell short.
Will once-daily fosamprenavir work with 100 mg of ritonavir?
Fosamprenavir/ritonavir ranks as one of several once-daily boosted protease inhibitor (PI) options sanctioned for treatment-naive people, at a dose of 1400/200 mg. But will 1400/100 mg once daily work just as well--and with fewer side effects? Three trials presented in Glasgow addressed that question, and all yielded generally favorable findings. But questions remain about how the 1400/100-mg dose will affect lipids over the long term.
Earlier research charted 38% lower amprenavir troughs in HIV-negative volunteers taking fosamprenavir with 100 mg of ritonavir once daily than in those taking 200 mg of ritonavir [1]. (The body metabolizes fosamprenavir to amprenavir.) But those troughs remained 6 times higher than the protein binding-adjusted 50% inhibitory concentration for nonmutant ("wild-type") virus. To see how the 100-mg boost compared with the 200-mg kicker, Duke University's Charles Hicks and colleagues at other US sites randomized 115 previously untreated people to 100 or 200 mg of ritonavir plus 1400 mg of fosamprenavir with fixed-dose abacavir/3TC (epzicom, Kivexa)--all given once daily [2].
Pretreatment viral loads were higher and CD4 counts lower in the 200-mg arm (medians of 83,200 versus 46,500 copies, and 179 versus 259 cells). Twenty-six of 57 people (46%) assigned to 200 mg of ritonavir and 20 of 58 (34%) assigned to 100 mg had a pretreatment load above 100,000 copies. The trial will run for 48 weeks. In the 24-week interim analysis presented in Glasgow, virologic response to the 100-mg booster looked better than response to the higher ritonavir dose (Table 1). Hicks and coworkers advised cautious interpretation of those results, however, because baseline numbers favored the 100-mg group. Average CD4 counts rose from 242 to 368 in the 100-mg arm and from 211 to 353 in the 200-mg arm.
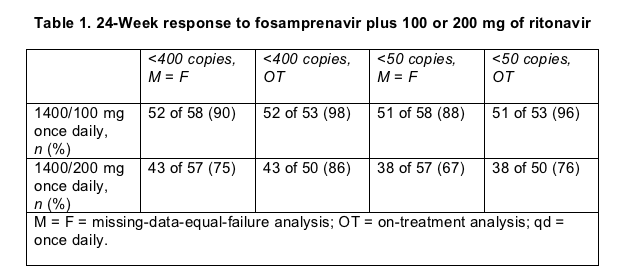
Five people (9%) dropped out of the 100-mg group before week 24, 1 because of side effects, 1 for a protocol violation, and 3 who stopped coming back for visits. Six people (11%) quit the 200-mg group, 1 for virologic failure, 2 because of side effects, and 3 who stopped keeping appointments. Grade 2 to 4 side effects were roughly equivalent in the two arms--affecting 36% taking 100 mg of ritonavir and 40% taking 200 mg. Diarrhea seemed more common with 200 mg of ritonavir, troubling 10 people (18%) versus 6 (10%) taking 100 mg.
A small surprise emerged in this analysis: the groups did not differ in fasting lipid levels after 24 weeks of therapy. Lipids were equivalent before treatment in the two groups, and fasting total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides rose similar degrees with the two boosting doses. Whether that means the two ritonavir doses have the same effect on lipids when given with once-daily fosamprenavir remains unclear. Early lipid climbs in HIV-infected people starting their first antiretrovirals may reflect a return to normal levels, a phenomenon observed in earlier studies.
Comparing 1400/100 mg of fosamprenavir/ritonavir once daily with 300/100 mg of atazanavir/ritonavir once daily--both with tenofovir DF/emtricitabine (TDF/FTC)--another group of US researchers graphed similar lipid changes with both regimens through 24 weeks, except for triglycerides [3]. Median pretreatment triglycerides measured 116 mg/dL in the fosamprenavir arm and 127 mg/dL in the atazanavir arm. Medians rose 44 mg/dL (38%) with fosamprenavir and 6 mg/dL (5%) with atazanavir. Virologic responses were similar in the two arms at 24 weeks.
Kimberly Smith (Rush University Medical Center, Chicago) and colleagues randomized 106 treatment-naive people to one of the two regimens, planning a 48-week follow-up with a primary endpoint of the proportion with fewer than 50 copies at that point. The groups matched well in pretreatment viral load (median 4.88 log or about 76,000 copies in both groups) and CD4 count (medians of 161 in the fosamprenavir group and 188 in the atazanavir group). About 20% in each group had AIDS when the trial began.
A planned 24-week missing-data-equal-failure analysis determined that 89% in each study arm had a viral load under 400 copies, while sub-50-copy rates measured 79% with fosamprenavir and 83% with atazanavir. Average CD4s climbed 126 cells with fosamprenavir and 155 cells with atazanavir. Four people dropped out of the fosamprenavir group, 1 because of nonresponse and 1 because of rash. Three people left the atazanavir group, 1 because of Kaposi sarcoma.
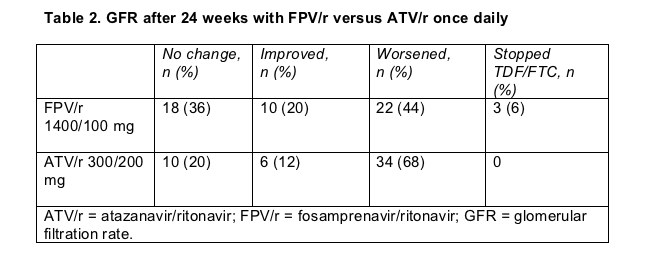
Grade 2 to 4 side effects affected 28 people (53%) taking atazanavir/ritonavir (including 22 bilirubin rises) and 6 (11%) taking fosamprenavir/ritonavir. Glomerular filtration rate, a measure of kidney function, got worse in more people taking atazanavir than in those taking fosamprenavir (Table 2). But the 3 people who had to stop TDF/FTC for protocol-defined GFR changes were all taking fosamprenavir/ritonavir.
A single-center, single-arm Belgian study charted good 24- and 48-week responses to fosamprenavir/ritonavir at 1400/100 mg once daily [4]. As in the fosamprenavir-versus-atazanavir trial [3], people taking fosamprenavir/ritonavir in the Belgian study had a sizeable triglyceride jump in the first months of therapy, but that gain appeared to level off in the normal range among people who took the drugs for 48 weeks. (That may not be the case with a 200-mg ritonavir booster, as results of the following study suggest [5].)
Stephane De Wit and colleagues from Saint-Pierre University Hospital in Brussels prospectively monitored 93 treatment-naive people, 50 of them (54%) African, for 24 to 48 weeks after they started fosamprenavir/ritonavir with TDF/3TC (64 people) or TDF/FTC (29 people) [4]. Median pretreatment viral load measured 87,600 copies (interquartile range [IQR] 27,350 to 100,000 copies), and median baseline CD4 count stood at 154 cells (IQR 63 to 259).
After 24 weeks 83% of study participants had a viral load below 50 copies and 92% were under 400 copies. Median CD4s climbed by 127 cells. Among 34 people who reached 48 weeks of follow-up, 83% had a sub-50 load and 94% had a sub-400 load. Median CD4 gain at week 48 measured 199 cells. So far 7 people stopped the regimen, 5 because of toxicity (4 gastrointestinal problems and 1 rash), and 2 because they switched to a two-pill-daily regimen. Triglycerides climbed 37.5% through 24 weeks but remained in the normal range at 121 mg/dL. Among people who have taken fosamprenavir/ritonavir for 48 weeks, median triglycerides stand at 106 mg/dL, a 20.5% rise from baseline.
Grade 3 or 4 triglyceride leaps affected 24 of 213 people (11%) who took fosamprenavir/ritonavir at a once-daily dose of 1400/200 mg for 180 weeks [5] in an extension of a trial comparing that dose to twice-daily nelfinavir in previously untreated people [6]. It is worth noting that 6 people (3%) had triglyceride jumps between treatment weeks 48 and 180, a finding underlining the importance of continued lipid monitoring during antiretroviral therapy. Sixteen people (8%) left the extension study before week 180 because of side effects or other complications, but the authors did not spell out individual causes of drug discontinuation. Abacavir/3TC was the nucleoside backbone in the original 48-week trial, and 88% continued those drugs in the extension phase.
Up-front tipranavir can't match lopinavir
An international trial pitting first-line tipranavir/ritonavir at two different doses against lopinavir/ritonavir for 60 weeks found the higher tipranavir/ritonavir dose (500/200 mg twice daily) too toxic and the lower dose (500/100 mg twice daily) too weak to merit consideration for treatment-naive people [7]. With so many once-daily PI options available for starting regimens, whether a different twice-daily tipranavir/ritonavir dose would fare better seems a moot question.
David Cooper (National Centre in HIV, Sydney) and confreres in Argentina and Thailand randomized 558 previously untreated people to one of the two tipranavir/ritonavir doses or to standard-dose lopinavir/ritonavir plus 3TC and TDF. Median pretreatment viral load stood just above 100,000 copies and median CD4 count just above 200. While 11% of study participants had fewer than 50 CD4 cells when entering the trial, 16% had hepatitis C infection.
About 85% of participants completed 48 weeks of treatment. At that point the proportion of people who had a viral load below 50 copies without changing antiretrovirals was 69.2% in the lopinavir arm, 66.7% in the tipranavir/ritonavir-200 arm, and 65.8% in the tipranavir/ritonavir-100 arm. Those results suggested the three regimens are equivalent. But 15 people in the three arms reached their first sub-50 load at week 48. Since the trial's primary endpoint mandated a confirmed sub-50 load for the between-arm comparisons, Cooper and colleagues went on to compare 60-week sub-50 results. Statisticians reckoned that after 60 weeks tipranavir/ritonavir-100 did not meet predetermined criteria that would establish it as noninferior to lopinavir/ritonavir. In plain English the 500/100-mg twice-daily dose was virologically inferior to lopinavir/ritonavir in pushing viral loads under 50 copies.
In the same 60-week analysis the 500/200-mg dose of tipranavir/ritonavir did prove virologically noninferior to lopinavir/ritonavir--but at the cost of more toxicity. Rates of investigator-defined drug-related "adverse events" measured 55% for lopinavir/ritonavir, 62.5% for tipranavir/ritonavir-100, and 69% for tipranavir/ritonavir-200. While 3% stopped lopinavir/ritonavir because of side effects, 7% stopped 500/100 mg of tipranavir/ritonavir and 10% stopped 500/200 mg. Significantly fewer people taking the higher tipranavir/ritonavir dose (82%) than taking the lower dose (94%) or taking lopinavir (97%) remained free of grade 3-4 alanine aminotransferase elevations through 48 weeks (P = 0.001).
Cooper and colleagues concluded that "at the doses tested in this trial, tipranavir/ritonavir cannot be recommended for antiretroviral-naive patients infected with wild-type HIV-1."
SQV/r versus LPV/r: an early look that's too early?
Saquinavir/ritonavir appeared to match lopinavir/ritonavir as a first-line boosted PI, but the preliminary and partial nature of the report stand in the way of any firm conclusions [8]. GEMINI trial researchers recruited 337 treatment-naive people at 38 sites in the US, Puerto Rico, Canada, France, and Thailand and randomized them to saquinavir/ritonavir (1000/100 mg twice daily) or standard-dose lopinavir/ritonavir with a nucleoside backbone of fixed-dose TDF/FTC (Truvada). Primary endpoints are the number and percentage of people in each arm with a load below 50 copies at week 48. The preliminary analysis presented in Glasgow by Jihad Slim (St. Michael's Medical Center, Newark) involved only the first 150 people to reach week 24.
Before treatment began the 24-week subset had a median viral load just above 100,000 copies, though 75% in the lopinavir arm versus 58% in the saquinavir arm had a starting load atop the 100,000-copy mark. Pretreatment CD4 counts averaged 134 in the saquinavir group versus 121 in the lopinavir group, and 32% starting saquinavir versus 40% starting lopinavir had fewer than 50 CD4s. A higher proportion of people in the lopinavir arm (37%) than in the saquinavir arm (28%) already had an AIDS diagnosis.
A 24-week intent-to-treat analysis found no significant difference between the two treatment arms in percent with a viral load below 400 copies (83.6% lopinavir versus 80.6% saquinavir) or below 50 copies (75.3% lopinavir versus 69.4% saquinavir). On-treatment analysis trends paralleled these 24-week intent-to-treat rates. At this point of follow-up the GEMINI team counted 5 virologic failures in the saquinavir group (6.8%) versus 2 (2.6%) in the lopinavir group. As in other studies of first-line boosted PIs, no new PI mutations emerged with any of these failures. The lopinavir group gained slightly more CD4 cells than the saquinavir group (154 versus 140), a nonsignificant difference at this point.
Whereas 58% in the lopinavir group had one or more side effects in 24 weeks, 48% in the saquinavir group did. Gastrointestinal problems including nausea, vomiting, and diarrhea affected 23% taking lopinavir and 14% taking saquinavir. Yet discontinuations proved similar in the two groups-19% taking saquinavir and 17% taking lopinavir. One person without known pretreatment liver ailments stopped lopinavir/ritonavir because of liver failure and later died. Two serious drug-related problems arose in the saquinavir group, hypokalemia and an acute psychotic episode.
Fasting cholesterol and triglyceride elevations were similar in the two study arms before treatment began, but both rose more often in the lopinavir group (Table 3).
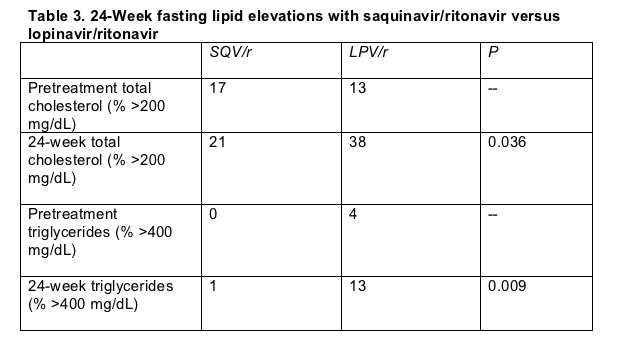
All these results must be considered preliminary because they represent fewer than half of the study population, and because the lopinavir group appeared to have more advanced HIV infection when treatment began. An earlier comparison of the same doses of saquinavir/ritonavir and lopinavir/ritonavir in 324 patients--about one third of them treatment naive--found a faster time to treatment failure in the saquinavir arm at week 48.[9] The GEMINI researchers could not present a time-to-treatment-failure analysis because of the limited number tracked to week 24. In the earlier trial [9] significantly more people discontinued treatment by week 48 in the saquinavir arm (29%) than in the lopinavir arm (13%) (P = 0.001).
Triglyceride jumps with atazanavir/ritonavir plus abacavir/3TC
A first-line regimen many would predict to be relatively free of unpleasant lipid surprises--atazanavir/ritonavir plus fixed-dose abacavir/3TC--turned out to drive median triglycerides above the National Cholesterol Education Program (NCEP) cutoff in 24 weeks, according to a report by Richard Elion (George Washington University, Washington, DC) and SHARE study colleagues.[10] The 48-week pilot trial signed up 112 previously untreated people with a median viral load of 5.06 log (just over 100,000 copies) and a median CD4 count of 208 (54% at or above 200 CD4s).
In the first 24 weeks, 5 people made a permitted switch from atazanavir to fosamprenavir because of side effects (hyperbilirubinemia and scleral icterus) and 8 made a permitted swap of abacavir/3TC for AZT/3TC because of hypersensitivity to abacavir. Seven people left the study, 2 because of side effects. A 24-week missing-data-equal-failure analysis figured that 85% had a viral load below 50 copies and 90% below 400 copies. One person failed to reach a viral load under 400 copies by week 24, and 1 had a confirmed rebound after going under 400. The median CD4 count climbed 133 cells.
While 21% of study participants had an ACTG-defined grade 1 cholesterol jump in the first 24 weeks, 5% had a grade 2 leap. During that time 17% had a grade 1 triglyceride elevation, 4% a grade 2 elevation, and 1% a grade 3 hike. Total cholesterol rose 17% and LDL cholesterol 7%, but both median spurts remained below NCEP III cutoffs of 200 and 130 mg/dL. Triglycerides climbed 30% during the first 24 weeks, and median levels pushed through the NCEP III safety ceiling of 150 mg/dL. Among 61 people with pretreatment triglycerides below 150 mg/dL, 28 (46%) edged up into a higher NCEP bracket. Of 32 people with triglycerides above 150 mg/dL at baseline, 12 (37.5%) took at least one step up the NCEP ladder.
Elion and colleagues surmised that ritonavir boosting--with the standard 100 mg once daily--may explain the disquieting triglyceride numbers.
Another induction-maintenance scheme falls short
A 72-week induction-maintenance trial raised more questions than it answered about this troubled strategy for people starting their first antiretrovirals [11]. In this 220-person Spanish trial, TRIZEFAL, surprisingly high proportions of people did not reach a viral load below 50 copies in 24 weeks despite taking a zesty four-drug regimen--either efavirenz or lopinavir/ritonavir plus Trizivir (fixed-dose AZT, 3TC, and abacavir). As a result many patients could not enter the trial's 48-week maintenance phase, which relied on Trizivir alone.
A high early dropout rate also affected an earlier US trial of induction with Trizivir plus efavirenz and subsequent maintenance with Trizivir [12]. In that trial 37% quit the study before the maintenance phase began at week 48, but enough people stayed in the trial to support statistical calculations after the maintenance phase. Josep Mallolas (Hospital Clinic, Barcelona) said TRIZEFAL had enough power to compare study groups after maintenance, but he did not present the power calculations.
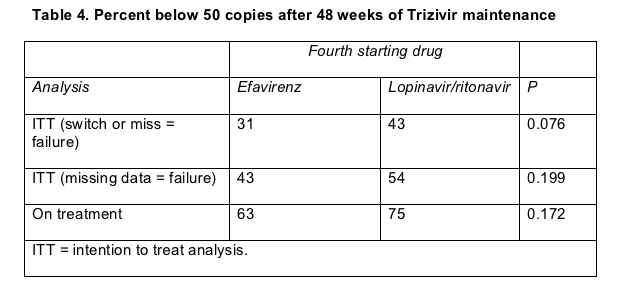
During TRIZEFAL's 48-week maintenance phase, more people who started induction with lopinavir than with efavirenz reached the primary endpoint--a load below 50 copies at week 72--in three different analyses (Table 4), though the difference between the lopinavir and efavirenz arms always fell shy of statistical significance. In the two intent-to-treat analyses, half or fewer patients (depending on the starting regimen) maintained a sub-50 load from week 24 through week 72 on Trizivir, not an encouraging result. Kaplan-Meier analysis of time to treatment failure also suggested a (nonsignificant) trend favoring induction with lopinavir/ritonavir (P = 0.076).
In the US Trizivir/efavirenz induction-maintenance trial [12], Martin Markowitz and colleagues tallied 16 maintenance-phase failures in the Trizivir-only arm and 8 in the continued Trizivir/efavirenz group, a difference that did not reach statistical significance (P = 0.134). TRIZEFAL did not have a control arm of people who reached an undetectable load in the induction phase then continued efavirenz or lopinavir with Trizivir in the maintenance phase. So it's impossible to determine whether Trizivir maintenance did as well as continued four-drug therapy would have. The intent-to-treat sub-50-copy results after 48 weeks of maintenance and 72 weeks of total therapy are not as good as those seen in some trials in which people continued a standard efavirenz or lopinavir regimen that long. In ACTG A5142, for example, 77% taking lopinavir/ritonavir plus two nucleosides and 89% taking efavirenz plus two nucleosides had fewer than 50 copies at week 96 [13].
Before induction therapy began, the efavirenz and lopinavir groups had similar median CD4 counts (201.5 in the efavirenz arm and 205 in the lopinavir arm) and median viral loads (144,233 copies and 160,000 copies). About one quarter of each group had AIDS when the trial started.
Only 54 people (52%) in the efavirenz group and 45 (43%) in the lopinavir group met the criterion needed to continue into the maintenance phase--a 24-week load below 50 copies. Most dropouts before 24 weeks reflected "adverse events" (side effects or clinical problems), including 33 people taking efavirenz (66% of dropouts) and 26 taking lopinavir/ritonavir (57% of dropouts). Three people in each study arm did not proceed to the maintenance phase because of virologic failure.
Of the adverse event-related dropouts in the efavirenz group, 15 resulted from hypersensitivity reactions attributed to abacavir, 3 from gastrointestinal side effects, 7 from neurologic problems probably caused by efavirenz, and 5 from hematologic abnormalities. Of the 25 adverse event-related dropouts in the lopinavir arm, 10 resulted from hypersensitivity reactions, 8 from gastrointestinal problems, 1 from neurologic problems, and 5 from hematologic abnormalities.
After 12 months of study in the Mallolas trial, people who started with lopinavir/ritonavir gained significantly more CD4 cells than those who started with efavirenz (P = 0.034), a result mirroring better CD4 gains with lopinavir than efavirenz (plus 3TC and AZT, d4T, or tenofovir) in ACTG 5142 [13]. But at earlier and later points of follow-up in the induction-maintenance trial, CD4 gain differences between the lopinavir and efavirenz arms lacked statistical significance.
Mark Mascolini writes about HIV infection (markmascolini@earthlink.net).
References
1. Ruane P, Luber A, Wire MB, et al. Plasma amprenavir pharmacokinetics and safety following co-administration of fosamprenavir with a reduced ritonavir dose once daily (COL 10053). 44th ICAAC. October 30-November 2, 2004. Washington, DC. Abstract A-449.
2. Hicks C, DeJesus E, Sloan L, et al. Efficacy and safety of once-daily boosted fosamprenavir/ritonavir with abacavir/lamivudine fixed dose combination in antiretroviral naive HIV-1 infected patients: 24-week results from COL100758. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract P2.
3. Smith K, Weinberg W, DeJesus E, et al. Once-daily boosted fosamprenavir (FPV/r) or atazanavir (ATZ/r) with tenofovir/emtricitabine in antiretroviral naive HIV-1 infected patients: 24-week results from COL103952 (ALERT). 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract P1.
4. De Wit S, Poll B, Necsoi C, Clumeck N. Fosamprenavir boosted with a single 100 mg capsule of ritonavir as part of a once daily first line regimen in naive patients. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract P17.
5. Bellos N, Clumeck N, Bleiber G, et al. Sustained virologic and immunologic response over 180 weeks in antiretroviral therapy-naive subjects receiving fosamprenavir/ritonavir QD. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract P13.
6. Gathe JC, Ive P, Wood R, et al. SOLO: 48-week efficacy and safety comparison of once-daily fosamprenavir/ritonavir versus twice daily nelfinavir in naive HIV-1-infected patients. AIDS. 2004;18:1529-1537.
7. Cooper D, Zajdenverg R, Ruxrungthm K, Chavez L. Efficacy and safety of two doses of tipranavir/ritonavir versus lopinavir/ritonavir-based therapy in antiretroviral-na´ve patients: results of BI 1182.33. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006.Glasgow. Abstract PL13.4.
8. Slim J, Avihingsanon A, Ruxrungtham K, et al. Saquinavir BID vs lopinavir plus emtricitabine/tenofovir QD in ARV-naive HIV-1 infected patients: GEMINI study. 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract PL2.5.
9. Dragsted UB, Gerstoft J, Youle M, et al. A randomized trial to evaluate lopinavir/ritonavir versus saquinavir/ritonavir in HIV-1-infected patients: the MaxCmin2 trial. Antivir Ther. 2005;10:735-743.
10. Elion R, DeJesus E, Sension M, et al. Once-daily abacavir/lamivudine and ritonavir-boosted atazanavir in antiretroviral naive HIV-1 infected subjects: 24-week analysis from COL102060 (SHARE). 8th International Congress on Drug Therapy in HIV Infection. November 12-16, 2006. Glasgow. Abstract 10.
11. Mallolas J, Penaranda M, Domingo P, et al. Induction maintenance antiretroviral therapy with Trizivir plus either efavirenz or lopinavir/r: results of a multicenter randomised clinical trial at 72 weeks. 8th International Congress on Drug Therapy in HIV Infection, November 12-16, 2006, Glasgow. Abstract 2.3.
12. Markowitz M, Hill-Zabala C, Lang J, et al. Induction with abacavir/lamivudine/zidovudine plus efavirenz for 48 weeks followed by 48-week maintenance with abacavir/lamivudine/zidovudine alone in antiretroviral-naive HIV-1-infected patients. JAIDS. 2005;39:257-264.
13. Riddler SA, Haubrich R, DiRienzo G, et al. A prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV infection--ACTG 5142. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THLB0204.
|
| |
|
 |
 |
|
|