 |
 |
 |
| |
Fosamprenavir/r 1400/100 Once Daily Looks Promising
|
| |
| |
Excerpted from: Boosted PIs and Other First-Line Strategies
Glasgow Meeting: Part 4
8th International Congress on Drug Therapy in HIV Infection
November 12-16, 2006, Glasgow
Mark Mascolini
Four trials presented at the Glasgow meeting offered insights into first-line fosamprenavir/ritonavir, and three of them appraised 100 mg of ritonavir once daily as the boosting dose. Other investigators sized up the merits of first-line boosted tipranavir or saquinavir, fixed-dose abacavir/lamivudine (3TC) plus atazanavir/ritonavir once daily, and an induction-maintenance scheme that fell short.
Will once-daily fosamprenavir work with 100 mg of ritonavir?
Fosamprenavir/ritonavir ranks as one of several once-daily boosted protease inhibitor (PI) options sanctioned for treatment-naive people, at a dose of 1400/200 mg. But will 1400/100 mg once daily work just as well--and with fewer side effects? Three trials presented in Glasgow addressed that question, and all yielded generally favorable findings. But questions remain about how the 1400/100-mg dose will affect lipids over the long term.
Earlier research charted 38% lower amprenavir troughs in HIV-negative volunteers taking fosamprenavir with 100 mg of ritonavir once daily than in those taking 200 mg of ritonavir [1]. (The body metabolizes fosamprenavir to amprenavir.) But those troughs remained 6 times higher than the protein binding-adjusted 50% inhibitory concentration for nonmutant ("wild-type") virus. To see how the 100-mg boost compared with the 200-mg kicker, Duke University's Charles Hicks and colleagues at other US sites randomized 115 previously untreated people to 100 or 200 mg of ritonavir plus 1400 mg of fosamprenavir with fixed-dose abacavir/3TC (epzicom, Kivexa)--all given once daily [2].
Pretreatment viral loads were higher and CD4 counts lower in the 200-mg arm (medians of 83,200 versus 46,500 copies, and 179 versus 259 cells). Twenty-six of 57 people (46%) assigned to 200 mg of ritonavir and 20 of 58 (34%) assigned to 100 mg had a pretreatment load above 100,000 copies. The trial will run for 48 weeks. In the 24-week interim analysis presented in Glasgow, virologic response to the 100-mg booster looked better than response to the higher ritonavir dose (Table 1). Hicks and coworkers advised cautious interpretation of those results, however, because baseline numbers favored the 100-mg group. Average CD4 counts rose from 242 to 368 in the 100-mg arm and from 211 to 353 in the 200-mg arm.
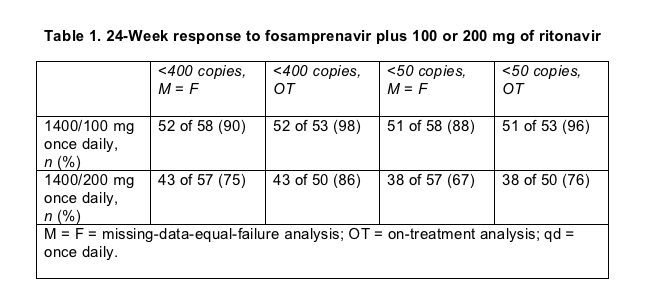
Five people (9%) dropped out of the 100-mg group before week 24, 1 because of side effects, 1 for a protocol violation, and 3 who stopped coming back for visits. Six people (11%) quit the 200-mg group, 1 for virologic failure, 2 because of side effects, and 3 who stopped keeping appointments. Grade 2 to 4 side effects were roughly equivalent in the two arms--affecting 36% taking 100 mg of ritonavir and 40% taking 200 mg. Diarrhea seemed more common with 200 mg of ritonavir, troubling 10 people (18%) versus 6 (10%) taking 100 mg.
A small surprise emerged in this analysis: the groups did not differ in fasting lipid levels after 24 weeks of therapy. Lipids were equivalent before treatment in the two groups, and fasting total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides rose similar degrees with the two boosting doses. Whether that means the two ritonavir doses have the same effect on lipids when given with once-daily fosamprenavir remains unclear. Early lipid climbs in HIV-infected people starting their first antiretrovirals may reflect a return to normal levels, a phenomenon observed in earlier studies.
Comparing 1400/100 mg of fosamprenavir/ritonavir once daily with 300/100 mg of atazanavir/ritonavir once daily--both with tenofovir DF/emtricitabine (TDF/FTC)--another group of US researchers graphed similar lipid changes with both regimens through 24 weeks, except for triglycerides [3]. Median pretreatment triglycerides measured 116 mg/dL in the fosamprenavir arm and 127 mg/dL in the atazanavir arm. Medians rose 44 mg/dL (38%) with fosamprenavir and 6 mg/dL (5%) with atazanavir. Virologic responses were similar in the two arms at 24 weeks.
Kimberly Smith (Rush University Medical Center, Chicago) and colleagues randomized 106 treatment-naive people to one of the two regimens, planning a 48-week follow-up with a primary endpoint of the proportion with fewer than 50 copies at that point. The groups matched well in pretreatment viral load (median 4.88 log or about 76,000 copies in both groups) and CD4 count (medians of 161 in the fosamprenavir group and 188 in the atazanavir group). About 20% in each group had AIDS when the trial began.
A planned 24-week missing-data-equal-failure analysis determined that 89% in each study arm had a viral load under 400 copies, while sub-50-copy rates measured 79% with fosamprenavir and 83% with atazanavir. Average CD4s climbed 126 cells with fosamprenavir and 155 cells with atazanavir. Four people dropped out of the fosamprenavir group, 1 because of nonresponse and 1 because of rash. Three people left the atazanavir group, 1 because of Kaposi sarcoma.
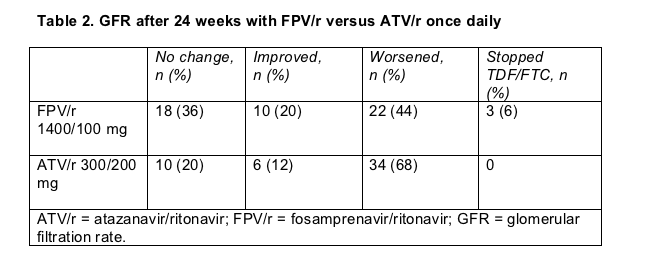
Grade 2 to 4 side effects affected 28 people (53%) taking atazanavir/ritonavir (including 22 bilirubin rises) and 6 (11%) taking fosamprenavir/ritonavir. Glomerular filtration rate, a measure of kidney function, got worse in more people taking atazanavir than in those taking fosamprenavir (Table 2). But the 3 people who had to stop TDF/FTC for protocol-defined GFR changes were all taking fosamprenavir/ritonavir.
A single-center, single-arm Belgian study charted good 24- and 48-week responses to fosamprenavir/ritonavir at 1400/100 mg once daily [4]. As in the fosamprenavir-versus-atazanavir trial [3], people taking fosamprenavir/ritonavir in the Belgian study had a sizeable triglyceride jump in the first months of therapy, but that gain appeared to level off in the normal range among people who took the drugs for 48 weeks. (That may not be the case with a 200-mg ritonavir booster, as results of the following study suggest [5].)
Stephane De Wit and colleagues from Saint-Pierre University Hospital in Brussels prospectively monitored 93 treatment-naive people, 50 of them (54%) African, for 24 to 48 weeks after they started fosamprenavir/ritonavir with TDF/3TC (64 people) or TDF/FTC (29 people) [4]. Median pretreatment viral load measured 87,600 copies (interquartile range [IQR] 27,350 to 100,000 copies), and median baseline CD4 count stood at 154 cells (IQR 63 to 259).
After 24 weeks 83% of study participants had a viral load below 50 copies and 92% were under 400 copies. Median CD4s climbed by 127 cells. Among 34 people who reached 48 weeks of follow-up, 83% had a sub-50 load and 94% had a sub-400 load. Median CD4 gain at week 48 measured 199 cells. So far 7 people stopped the regimen, 5 because of toxicity (4 gastrointestinal problems and 1 rash), and 2 because they switched to a two-pill-daily regimen. Triglycerides climbed 37.5% through 24 weeks but remained in the normal range at 121 mg/dL. Among people who have taken fosamprenavir/ritonavir for 48 weeks, median triglycerides stand at 106 mg/dL, a 20.5% rise from baseline.
Grade 3 or 4 triglyceride leaps affected 24 of 213 people (11%) who took fosamprenavir/ritonavir at a once-daily dose of 1400/200 mg for 180 weeks [5] in an extension of a trial comparing that dose to twice-daily nelfinavir in previously untreated people [6]. It is worth noting that 6 people (3%) had triglyceride jumps between treatment weeks 48 and 180, a finding underlining the importance of continued lipid monitoring during antiretroviral therapy. Sixteen people (8%) left the extension study before week 180 because of side effects or other complications, but the authors did not spell out individual causes of drug discontinuation. Abacavir/3TC was the nucleoside backbone in the original 48-week trial, and 88% continued those drugs in the extension phase.
|
| |
|
 |
 |
|
|