| |
Past HBV Viral Load as Predictor of Mortality and Morbidity from HCC and Chronic Liver Disease in a Prospective Study
|
| |
| |
The American Journal of Gastroenterology
Volume 101 Page 1797 - August 2006
Gang Chen, Ph.D.1, Wenyao Lin, M.D.2, Fumin Shen, M.D.3, Uchenna H. Iloeje, M.B.B.S., M.P.H., F.A.C.P.4, W. Thomas London, M.D.1, and Alison A. Evans, Sc.D.1
1Fox Chase Cancer Center, Philadelphia, Pennsylvania; 2Haimen City Center for Disease Control, Haimen City, China; 3School of Public Health, Fudan University, Shanghai, China; and 4Global Epidemiology and Outcomes Research, Pharmaceutical Research Institute, Bristol-Myers Squibb, Wallingford, Connecticut
What Is Current Knowledge
- Risk prediction in chronic hepatitis B virus (HBV) is difficult.
- Hepatocellular carcinoma (HCC) and chronic liver disease risks vary widely.
- Molecular methods have shown promise.
What Is New Here
- High viral load is a strong predictor of liver-specific mortality.
- Past viral load predicts current liver disease severity.
- Viral load a useful tool in determining risk level in HBV.
..... In this study we report the results of a long-term prospective study in HBV-infected Chinese adults from the Haimen City cohort.... In this prospective study we have demonstrated the prognostic significance of quantitative HBV viral load for mortality from HCC and CLD over an 11-yr follow-up. In addition, high HBV viral load over a decade earlier is shown to be associated with the severity of current liver disease. A growing number of studies support the clinical and prognostic significance of quantitative HBV viral load measurements (21-24), but few such as the recently published R.E.V.E.A.L-HBV study (13) have had the advantage of the large numbers of subjects and long periods of follow-up that are available to us..... demonstrated by our study and the results of the R.E.V.E.A.L.-HBV study is that HBV DNA viral load established using a sensitive methodology can predict those CHB-infected persons that are at risk of liver disease complications.... it is important to explore other ways of predicting patients at risk even more precisely and develop treatment strategies beyond antiviral therapy to prevent morbidity and mortality from HCC and CLD in high-risk populations....
....In HBV-infected individuals with cirrhosis or advanced fibrosis, Liaw et al. have shown that antiviral therapy that reduces viral load can decrease the risk of progression and HCC, with a median follow-up time of 32 months (29). More recent data presented at the 56th annual meeting of the American Association for the Study of Liver Disease suggest that in noncirrhotic subjects, treatment with antiviral therapy slows liver disease progression (30). The implications of our findings are that beneficial effects of viral load reduction may be evident over a longer time period and the benefits may not be confined to those with severe liver disease.....
.... the conclusions to be drawn from our mortality analysis are clear and relatively straightforward. Viral load was a strong predictor of liver-related mortality over an 11-yr follow-up period. Visual inspection of the survival curves suggests that the elevated risk is essentially constant throughout the follow-up period, and this conclusion is borne out by formal statistical testing. The cumulative impact of this elevated risk is substantial -more than 20% of those with high viral loads (>100,000 c/ml) had died of HCC or CLD by the end of the follow-up period. Moreover, a considerable proportion of the survivors evaluated in 2003 had severe liver disease, and the severity of their disease was strongly associated with the viral load of 11 yr earlier. Among the survivors who had a high viral load in the past, 29% now have severe liver disease and/or HCC. Thus, it appears that the elevated risk of liver-related mortality will continue to affect these individuals. Those with low or undetected viral load at study entry, however, were not spared the harmful effects of HBV infection. Seventeen percent of the survivors had severe liver disease or HCC after 11 yr of follow-up, and 2% had died of CLD or HCC in the interim......
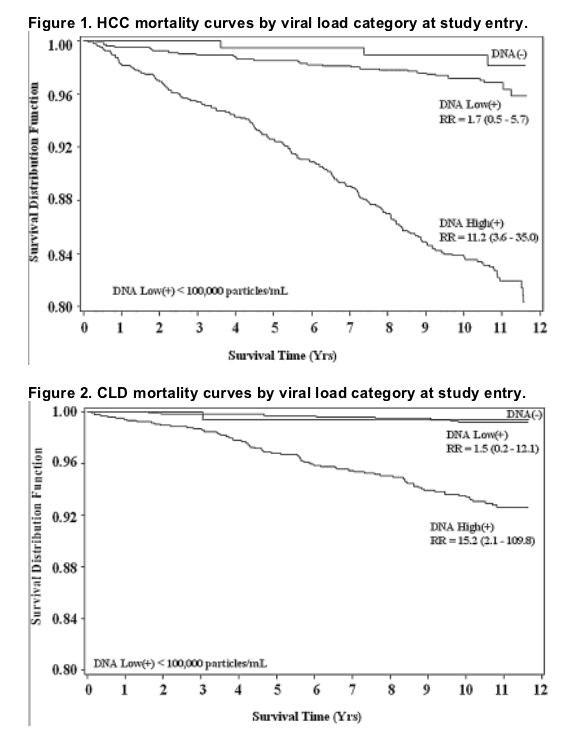
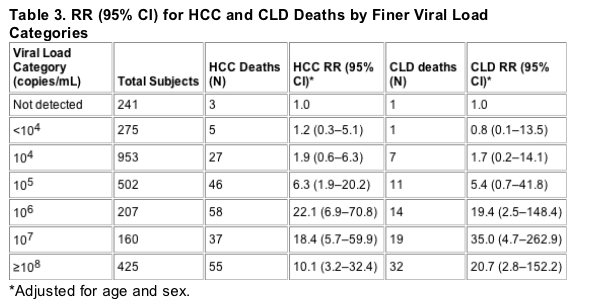
Figures 1 and 2 show survival curves from study entry to the end of the follow-up period for HCC mortality and CLD mortality, respectively. The increased risk associated with high viral load appears to be present throughout the follow-up period for both categories of mortality......(note from Jules Levin: 100,000 c/ml curve widely separated from 1600 to 10,000 c/ml curve; undetectable separated but not as by as wide a margin from low viral load 1,600 to 10,000 c/ml. In firgure 2 undetectable curve not separated from low viral load, but low and high viral load curves are widely separated).
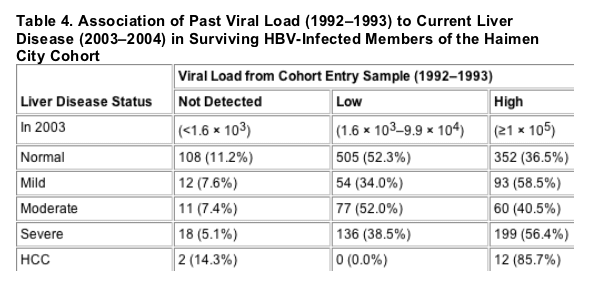
In order to examine evidence for a threshold effect, the estimates in Table 3 use finer viral load categories than the main analysis. The estimates are consistent with an increase in risk of HCC up to 106 copies/mL (1 million c/ml), after which risk plateaus or slightly decreases. For CLD -clinical liver disease-, the peak occurs at 107 copies/mL (10 million c/ml), with a similar plateau. Because of the small numbers of HCC or CLD deaths in the lowest viral load categories, it was not feasible to formally assess evidence for a threshold. But, consistent with the main analysis, this analysis suggests that mortality increases with increasing viral load, with very wide confidence intervals at the lowest levels. Adjustment for HBeAg status (not shown) produced similar results with somewhat lower RRs, as in the main analysis.
In order to estimate the OR of liver disease among different viral load categories, the Mild and Moderate liver disease groups were combined and the Severe and HCC groups were combined. All OR estimates were controlled for age and sex. Compared with those with undetected DNA, the OR for Severe liver disease for the low viral load category was 1.3 (95% CI 0.8-2.1) and for high viral load 2.7 (95% CI 1.6-4.5), ptrend< 0.001. For Mild/Moderate liver disease, the low viral load OR was 1.1 (0.6-1.7) and high viral load was 1.7 (1.0-2.8), ptrend = 0.0013. Exclusion of HCC from the severe CLD group changed these estimates only slightly.
ABSTRACT
BACKGROUND AND AIMS: In a prospective cohort study with 11 yr of follow-up, we assessed the relationship between past hepatitis B virus (HBV) viral load and mortality. Surviving cohort members were evaluated for current liver disease.
METHODS: We measured HBV viral load by real-time polymerase chain reaction on stored samples from cohort entry (1992-1993) in 2,763 hepatitis B surface antigen (HBsAg)-positive adults. Major end points were death from hepatocellular carcinoma (HCC) or chronic liver disease (CLD). There were 447 deaths. In the 1,683 survivors, we assessed severity of liver disease on a return visit in 2003. Viral load was divided into three categories: undetected (<1.6 _ 103 copies/mL); low titer (<105 copies/mL); and high titer (≥105 copies/mL).
RESULTS:
For HCC, there was a significant increase in mortality across viral load categories (ptrend< 0.001). Compared to the HBV undetected category, the relative risk (RR) for HCC mortality in the low viral load group was 1.7 (95% confidence interval [CI] 0.5-5.7) and 11.2 (3.6-35.0) in the high viral load group. For CLD mortality, the RRs were 1.5 (0.2-12.1) and 15.2 (2.1-109.8), respectively (ptrend< 0.001). The RR associated with high viral load did not change with increased follow-up time. In surviving cohort members evaluated for liver disease in 2003, there was also a significant association of viral load with disease severity.
CONCLUSION: In this prospective study, viral load is associated with increased mortality from HCC and CLD in HBV-infected subjects. Viral load may be a useful prognostic tool in HBV infection, and interventions aimed at its reduction should be explored.
Introduction
Chronic infection with the hepatitis B virus (HBV) is an established cause of liver-related morbidity and mortality. HBV has been designated as a human carcinogen (1). It is estimated that a significant proportion (15-40%) of chronic HBV (CHB)-infected persons will progress to a liver-related event such as cirrhosis or hepatocellular carcinoma (HCC) (2, 3). China is an area of high endemicity of CHB infection, with an estimated 112 million chronically infected persons (4).
Predicting who among CHB-infected persons is at the highest risk of progressing to liver damage and intervening appropriately early enough to modify their disease course continues to be a topic of debate (5, 6). The use of quantitative serum HBV viral load to monitor therapeutic progress has become standard for all clinical trials for HBV drugs in development and has also been correlated to other indicators of therapeutic success such as loss of hepatitis B e antigen (HBeAg), loss of hepatitis B surface antigen (HBsAg), and improvement of hepatic inflammation and fibrosis scores (7).
In CHB-infected persons, several cross-sectional studies have demonstrated an association between active viral replication and ongoing liver damage (8, 9). More recently, large-scale prospective studies have found a positive relationship between hepatocellular carcinoma (HCC) and HBeAg, a serum marker strongly associated with viremia (10, 11). There is now a growing body of evidence from prospective epidemiologic studies suggesting a very strong relationship between quantitative HBV viral load and risk of future cirrhosis and HCC (12-14).
We have published nested case-control studies from prospective cohorts in Haimen City, China and Senegal, West Africa, which have demonstrated increased risk of HCC in persons positive for HBV DNA, using real-time PCR to increase the sensitivity of viral load detection and quantitation (15). In this study we report the results of a long-term prospective study in HBV-infected Chinese adults from the Haimen City cohort. The impact of HBV viral load on mortality from liver-related causes (HCC and CLD), as well as the impact of past viral load on present liver disease are reported.
RESULTS
Mortality Analysis
Viral load and follow-up data were available for 2,763 individuals, all of whom were HBsAg-positive at study entry in 1992-1993. Table 1 shows demographic characteristics of this group and the prevalence of major HCC risk factors. Viral load and HBeAg status were assayed on stored samples collected at the time of cohort entry in 1992-1993. Among the 2,763 individuals tested, 241 (8.7%) were classified as undetected (<1.6 _ 103 copies/mL; 1,600 c/ml), 1,228 (44.4%) had low viral load (1.6 _ 103-9.9 _ 104 copies/mL; 1600 to 10,000 c/ml), and 1,294 (46.8%) had high viral load (≥1 _ 105 copies/mL; >100,000 c/ml). HBeAg test results were available for 2,662 individuals, and 1,173 (44.0%) of these were positive. HBeAg positivity was more frequent in those with higher viral loads: 54 of 224 (24.1%) with undetected viral load, 293 of 1,190 (24.6%) with low viral load, and 826 of 1,248 (66.2%) with high viral load.
We examined the univariate association of viral load categories to age and sex groups. The prevalence of undetected HBV DNA increased with each decade of age, from 6.4% in subjects <30 yr to 9.4% in those ≥60 yr, but this difference was not statistically significant (ptrend = 0.33). Women were more likely than men to have undetected HBV DNA (13.1% vs 5.9%, p< 0.001). There was a strong association of viral load category to current cigarette smoking status (p-trend< 0.001) and a history of clinical hepatitis (p-trend< 0.001). Viral load categories were not significantly different by occupational class (peasants vs nonpeasants), alcohol drinking, or a history of HCC in a first- or second-degree relative.
During the follow-up period, 447 deaths occurred in this group. As expected in a group of HBV-infected individuals, HCC and CLD were the dominant causes of death. The crude all-cause mortality rate was 2,069 per 105 person-years (py) for men and 933 per 105 py for women. HCC deaths were 195 (crude rate 1,176 per 105 py) in men and 36 (323 per 105 py) in women. There were 61 (368 per 105 py) CLD deaths in men and 24 (215 per105 py) in women. Non-liver-related deaths were 87 (525 per 105 py) in men and 44 (395 per 105 py) in women.
Table 2 shows the relative risk (RR) of death by cause category across the subgroups of viral load, with the HBV DNA undetected group as the reference group and adjusted for age and sex only and adjusted for age, sex, and HBeAg status. Adjustment for other HBV risk factors (occupation, smoking, alcohol drinking, clinical hepatitis, and family history of HCC) did not change these estimates significantly. For both HCC and CLD deaths, there was a significantly positive trend for higher RR with increasing viral load category, but the individual RR estimates were statistically significant only in the high viral load (≥105 copies/mL) category. For non-liver-related deaths, there was no significant increase in risk associated with viral load categories. Figures 1 and 2 show survival curves from study entry to the end of the follow-up period for HCC mortality and CLD mortality, respectively. The increased risk associated with high viral load appears to be present throughout the follow-up period for both categories of mortality.
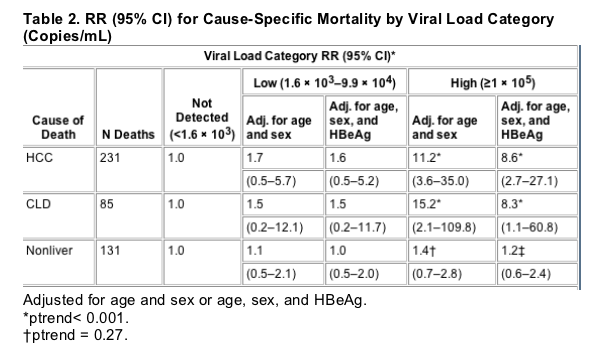
HBeAg positivity was also an independent risk factor for HCC and CLD mortality. Controlling for age, sex, and viral load, the RR (95% confidence interval [CI]) for HCC death was 1.5 (1.1-2.0) between HBeAg positives and negatives. For CLD deaths, the RR (95% CI) was 4.1 (2.2-7.5). HBeAg was not associated with a significantly increased risk for non-liver-related mortality, RR (95% CI) 1.3 (0.9-2.0). Controlling for HBeAg status reduced somewhat the RRs associated with high viral load in HCC and CLD mortality but did not substantially alter the estimates of risk in the low viral load category. Even when controlling for HBeAg status, there was significantly greater risk of HCC and CLD deaths in those with high viral load at baseline (Table 2).
To determine whether there is evidence for differences in risk related to viral load in HBeAg-positive versus HBeAg-negative individuals, we stratified on HBeAg status and estimated RRs separately for each stratum. For HBeAg-positives with undetected viral load as the baseline category, the RR (95% CI) for those with low viral load was 1.3 (0.2-10.4) and 7.5 (1.0-53.7) for high viral load. This stratum contained 77 HCC cases. For HBeAg-negatives, the estimates were 1.8 (0.4-7.6) and 9.3 (2.3-38.3), respectively, based on 142 cases. For CLD deaths, the number of cases was too small in the HBeAg-positive group (N = 13) to estimate the RRs. For the HBeAg-negative group (N = 67 cases), the RRs were 0.8 (0.1-7.6) for low viral load and 3.7 (0.5-30.1) for high viral load.
Evidence for Threshold
In order to examine evidence for a threshold effect, the estimates in Table 3 use finer viral load categories than the main analysis. The estimates are consistent with an increase in risk of HCC up to 106 copies/mL, after which risk plateaus or slightly decreases. For CLD, the peak occurs at 107 copies/mL, with a similar plateau. Because of the small numbers of HCC or CLD deaths in the lowest viral load categories, it was not feasible to formally assess evidence for a threshold. But, consistent with the main analysis, this analysis suggests that mortality increases with increasing viral load, with very wide confidence intervals at the lowest levels. Adjustment for HBeAg status (not shown) produced similar results with somewhat lower RRs, as in the main analysis.
Current Liver Disease among Survivors
Among the original 3,464 residents of these townships identified as HBsAg-positive in 1992-1993, 2,571 were alive and not lost to follow-up in June 2003. These individuals were contacted and offered the opportunity to attend a screening for additional HBV marker testing, liver function tests, ultrasounds of the liver, and physical examinations. Of those invited, 1,863 (72.5%) attended screening and 1,791 (96.1%) of these completed the entire evaluation. Using the criteria described in the Methods section, the current liver disease status of these individuals was classified as Normal (N = 1,059, 59.1%), Mild (N = 170, 9.5%), Moderate (N = 164, 9.2%), Severe (N = 381, 21.3%), and HCC (N = 17, 0.9%).
Table 1 shows the demographic characteristics, viral load, and HBeAg prevalence at baseline for this group. Because this group represents those who survived from 1992 to 2003, they would be expected to be somewhat healthier than the cohort as a whole. The prevalence of HBeAg by viral load category was 26 of 139 (18.7%) in the undetected group, 199 of 745 (26.7%) in the low viral load group, and 420 of 697 (60.3%) in the high viral load group. While viral load was correlated with HBeAg status, there were substantial numbers of HBeAg-negative individuals in the low (546 of 745, 73.3%) and high (277 of 697, 39.7%) viral load categories. Similarly, HBeAg prevalence from the cohort entry samples (1992-1993) was modestly correlated with the severity of liver disease in 2003. In those classified as Normal, 330 of 932 (35.4%) were HBeAg-positive. For the Mild, Moderate, and Severe+HCC groups, the comparable HBeAg prevalence numbers were 80 of 152 (52.6%), 55 of 144 (38.2%), and 180 of 353 (51.0%), respectively (ptrend< 0.001).
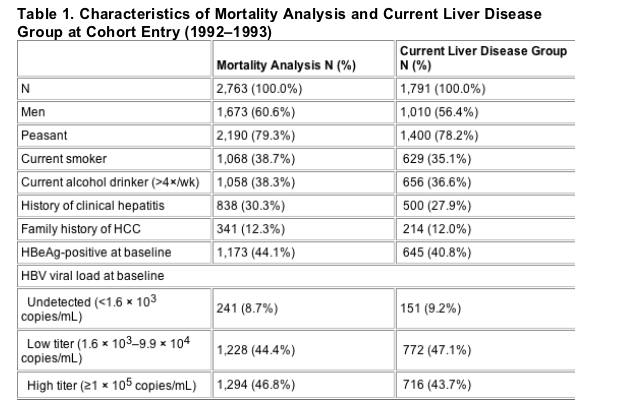
In order to evaluate the relationship between the surviving subjects' HBV viral load at cohort entry (1992-1993) and the prevalence of liver disease in 2003, we tested viral load in the 1,639 serum samples available from study entry for these 1,791 individuals, all of whom were HBsAg-positive at study entry. Table 4 shows the distribution of past viral load for current liver disease category.
The distribution across viral load categories also differed between men and women and by age group. In 724 women, 95 (13.1%) had undetected HBV DNA, 353 (48.8%) had low-titer HBV DNA, and 276 (38.1%) had high-titer HBV DNA. In 915 men, the distribution was 56 (6.1%), 419 (45.8%), and 440 (48.1%), respectively (ptrend< 0.001).
In order to estimate the OR of liver disease among different viral load categories, the Mild and Moderate liver disease groups were combined and the Severe and HCC groups were combined. All OR estimates were controlled for age and sex. Compared with those with undetected DNA, the OR for Severe liver disease for the low viral load category was 1.3 (95% CI 0.8-2.1) and for high viral load 2.7 (95% CI 1.6-4.5), ptrend< 0.001. For Mild/Moderate liver disease, the low viral load OR was 1.1 (0.6-1.7) and high viral load was 1.7 (1.0-2.8), ptrend = 0.0013. Exclusion of HCC from the severe CLD group changed these estimates only slightly.
DISCUSSION
In this prospective study we have demonstrated the prognostic significance of quantitative HBV viral load for mortality from HCC and CLD over an 11-yr follow-up. In addition, high HBV viral load over a decade earlier is shown to be associated with the severity of current liver disease. A growing number of studies support the clinical and prognostic significance of quantitative HBV viral load measurements (21-24), but few such as the recently published R.E.V.E.A.L-HBV study (13) have had the advantage of the large numbers of subjects and long periods of follow-up that are available to us.
The question of whether there is a threshold value below which viral load is not associated with an increased risk of adverse outcomes in HBV-infected persons is an important one. In our main analysis, we treated HBV viral load as a three-category variable because of the limitations of sample size, with the cutoff between low and high categories at 105 viral particles/mL. In category-by-category analyses, statistically significant effects were found only for those in the highest viral load category, but tests for trend were statistically significant and suggest a linear trend in the risk of adverse outcomes from undetected to low to high viral load. When we divided viral load into finer categories (shown in Table 3), confidence intervals were of course considerably wider. For HCC, risk appeared to peak in the 106 copies/mL group and then plateau thereafter. For CLD, the peak occurred at 107 copies/mL. The small numbers of HCC and CLD deaths with ≦104 copies/mL make it difficult to formally test for a threshold, but all estimated RRs for the categories ≥104 were greater than 1.0.
Data from the R.E.V.E.A.L.-HBV study suggest that the risk of liver complications begins to increase significantly at the level of 104 copies/mL, adjusted hazard ratio (95% CI) for HCC risk was 2.3 (1.1-4.9) (13), and for cirrhosis this was 2.5 (1.6-3.8) (14). Though results from our study and the R.E.V.E.A.L.-HBV study suggest a threshold of 104 copies/mL, others have found that a true threshold is difficult to establish. In a study of Chinese subjects from Hong Kong, a significant minority of subjects who had a liver complication and available HBV DNA data, 29% had undetectable HBV DNA levels (<200 copies/mL) (25). However, it is difficult to reach a definite conclusion based on the data presented in the Hong Kong study because 2,220 of the 2,332 subjects in the study (95%) had an HBV DNA level reported only at the last follow-up. Of the 110 subjects with paired HBV DNA samples and a hepatic complication, only 21 had their HBV DNA level reported before the development of a liver complication (range 0.2-8.25 months), and 80 subjects had their HBV DNA measured after complications occurred (range 0.41-193.5). There is still the need for further studies that have longitudinal data able to report on repeated HBV DNA results preceding any liver complication to answer the question of a threshold.
In this analysis we present measures of viral load from only one point in time, which may not give the complete picture of the relationship with severity of liver disease. Chu et al. (21) have shown that HBV DNA levels do fluctuate over time in most patient groups, particularly those with transient HBeAg loss. McMahon et al. have shown that transient changes in HBeAg status are independently associated with the risk of HCC (10). Measurement of viral load at multiple time points may offer the opportunity to distinguish immune-tolerant individuals with quiescent disease from those with more active immune response and consequent liver damage. Moreover, high viral load may be especially significant in subjects at older ages. Although our analyses were adjusted for age, the majority of our cohort was older than 40 yr of age at the time of entry, and it is difficult to generalize our findings to HBV-infected persons who are substantially younger.
Our study has some weaknesses, largely those inherent in a population-based rather than a clinic-based study. Information on causes of death was taken from death certificates. Though reports of liver-related deaths were investigated further through medical records and interviews of doctors and family members, death cause classification is subject to nondifferential misclassification that would bias our RR estimates toward the null. No measures of liver disease status are available from subjects at the inception of this cohort, and clearly the risk of mortality from CLD and HCC would be affected by liver status on study entry. We were able to evaluate current liver disease levels only among survivors, and the limitations of field research as well as ethical considerations meant that we could not perform the range of clinical tests, including liver biopsy, that would have given a much clearer indication of the extent of CLD. It is hoped that some of these questions can be answered in future studies with more complete information on liver disease status, and promising developments in the area of surrogate markers of liver disease that may reduce the need for liver biopsies might make such studies more feasible than they were at the time our cohort was assembled. Most of the recent literature on surrogate markers for viral hepatitis has focused on hepatitis C infection (26-28), and validation of these markers in HBV infection is needed.
Given these caveats, the conclusions to be drawn from our mortality analysis are clear and relatively straightforward. Viral load was a strong predictor of liver-related mortality over an 11-yr follow-up period. Visual inspection of the survival curves suggests that the elevated risk is essentially constant throughout the follow-up period, and this conclusion is borne out by formal statistical testing. The cumulative impact of this elevated risk is substantial-more than 20% of those with high viral loads had died of HCC or CLD by the end of the follow-up period. Moreover, a considerable proportion of the survivors evaluated in 2003 had severe liver disease, and the severity of their disease was strongly associated with the viral load of 11 yr earlier. Among the survivors who had a high viral load in the past, 29% now have severe liver disease and/or HCC. Thus, it appears that the elevated risk of liver-related mortality will continue to affect these individuals. Those with low or undetected viral load at study entry, however, were not spared the harmful effects of HBV infection. Seventeen percent of the survivors had severe liver disease or HCC after 11 yr of follow-up, and 2% had died of CLD or HCC in the interim.
Although Western antiviral medicines can be obtained in China, their use is uncommon in Haimen City (<1% of HBsAg-positive cohort members) because of high cost. In HBV-infected individuals with cirrhosis or advanced fibrosis, Liaw et al. have shown that antiviral therapy that reduces viral load can decrease the risk of progression and HCC, with a median follow-up time of 32 months (29). More recent data presented at the 56th annual meeting of the American Association for the Study of Liver Disease suggest that in noncirrhotic subjects, treatment with antiviral therapy slows liver disease progression (30). The implications of our findings are that beneficial effects of viral load reduction may be evident over a longer time period and the benefits may not be confined to those with severe liver disease. Though randomized clinical trials are needed to test this hypothesis, what has been demonstrated by our study and the results of the R.E.V.E.A.L.-HBV study is that HBV DNA viral load established using a sensitive methodology can predict those CHB-infected persons that are at risk of liver disease complications. As our understanding of the natural history of chronic HBV infection improves with the use of more sensitive and specific biological markers, it is also important to explore other ways of predicting patients at risk even more precisely and develop treatment strategies beyond antiviral therapy to prevent morbidity and mortality from HCC and CLD in high-risk populations.
METHODS
Study Population
The analysis used data and stored samples from a prospective cohort in Haimen City, located in Jiangsu Province, China. Previous publications have described the assembly of this cohort and previous analyses of HCC and all-cause mortality (16, 17). The study was reviewed and approved by the Institutional Review Board of Fox Chase Cancer Center, the Medical Ethics Review Group of Haimen City, and the Ethics Review Committee of the School of Public Health of Fudan University.
The initial cohort was assembled between February 1992 and December 1993 when study teams traveled to villages in each of the 35 townships of Haimen City to enroll subjects in a prospective cohort study. At entry, each subject completed a brief questionnaire and donated a 9.0-mL sample of blood by venipuncture. For the current analysis, enrolled subjects from nine randomly selected townships are included, all HBsAg-positive at cohort entry in 1992-1993 (N = 3,464), based on testing performed in the field at study entry. Twenty-nine subjects (0.8%) were withdrawn from the analysis because HBsAg positivity was not confirmed on later testing. In addition, 396 (11.4%) were lost to follow-up before 2004 and 276 (8.0%) had serum samples with insufficient quantity available for viral load testing.
The mortality analysis was performed on the data from 2,763 individuals who had complete follow-up and sufficient serum available for testing. The final analysis group differed from those excluded (N = 701) in being slightly older (mean age 41.9 ± 9.0 yr vs 40.1 ± 9.4 yr), less likely to be male (60.6% vs 69.3%, p< 0.001), more likely to be peasants (79.3% vs 61.2%, p< 0.001), less likely to drink alcoholic beverages >4 times per week (38.3% vs 45.5%, p< 0.001), and less likely to have a history of clinical hepatitis (30.3% vs 34.7%, p = 0.03). Older persons, women, and peasants are less likely than younger persons, men, and nonpeasants to leave Haimen City for periods of work in other areas, which accounts for much of this difference. The two groups did not differ significantly in the prevalence of current cigarette smoking (38.7% vs 42.2%, p = 0.08) and family history of HCC (12.3% vs 9.8%, p = 0.07). "Peasant" is the occupational designation given locally to persons whose main livelihood is from farm work in small personal or village land holdings.
Causes of death were initially ascertained from death certificates in yearly reviews of the vital status of all cohort members. In subjects whose death certificates indicated a liver-related death (HCC or CLD), additional information was sought to confirm this using medical records, interviews with village-, township-, and county-level doctors, and interviews of surviving family members.
Liver Disease Evaluation-2003
At study entry it was not possible to perform physical examinations or liver function tests on study participants. In 2003, the remaining 2,571 HBsAg-positive study participants who were not lost to follow-up were invited to return for evaluation of their current liver disease status. Each participant received blood testing for HBV markers, alanine aminotransferase (ALT), alphafetoprotein (AFP), an ultrasound of the liver, and a physical examination by a physician. No liver biopsies were performed. Of those invited, 1,863 (72.5%) subjects attended screening and 1,791 (96.1%) of these completed the entire evaluation.
Severity of liver disease was determined for each patient using criteria adapted for field conditions from the Dionysos Study (18, 19). Five levels of liver disease were defined a priori: Normal-no abnormalities on any test or exam except for HBV markers. Mild-abnormal ALT and/or AFP only. Moderate-physical findings or laboratory tests are abnormal but do not meet the criteria for probable fibrosis/cirrhosis or HCC. Severe-having at least two of the following: (1) spider nevi, scleral icterus, palmar erythema, ascites, hepatomegaly, or splenomegaly; (2) thrombocytopenia and/or prolonged (>2 s) prothrombin time; and (3) portal vein enlargement (>12 mm) on ultrasound. HCC-presence of a mass (>2 cm) on ultrasound and AFP >400 ng/mL.
Laboratory Methods
At the time of serum collection, samples were tested in Haimen for HBsAg using commercial immunoassay kits. Serum aliquots were stored at -20 C in Philadelphia, and retrieved in 2003 for viral load testing by real-time PCR. The method employed is similar to that we have previously published (15) and is described here briefly: HBV DNA in human sera was extracted along with a salmon sperm carrier by using a QIAamp DNA Blood Mini kit (Qiagen Inc., Valencia, CA) following the manufacturer's instructions. The prepared DNA samples were stored at -20 C until use. The TaqMan PCR assay was based on that published by Loeb et al. (20). The PCR primers were designed against a conserved region of the HBV genome, which included the genes encoding the X-protein and DNA polymerase, identical to those used by Loeb et al. The ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) was used for quantification of amplification at each cycle. The limit of detection for the assay was 20 copies/reaction or 1.6 _ 103 copies/mL. Samples were tested in duplicate, and repeated if there was a difference of >0.5 cycles between the replicates. Each PCR run contained negative controls and a standard curve of serially diluted HBV plasmids in duplicate.
Statistical Methods
All statistical analyses were performed using SAS V 8.2 (SAS Institute, Cary, NC). All statistical tests were two-sided. For the mortality analysis, Cox proportional hazards regression was used to calculate hazard ratios, with control for covariates as noted in the text. In the current liver disease analysis, nominal logistic regression models were fit in order to estimate odds ratios (ORs) for each category of liver disease severity without assuming a linear trend. Similarly, terms for viral load category were fit separately.
|
|
| |
| |
|
|
|