| |
Consensus Interferon in IFN/RBV Non-responders
|
| |
| |
Treatment with daily consensus interferon (CIFN) plus ribavirin in non-responder patients with chronic hepatitis C: A randomized open-label pilot study
Journal of Hepatology
Volume 44, Issue 2, Pages 291-301 (February 2006)
Markus Cornberga, Johannes Hadema, Eva Herrmannb, Frank Schuppertc, Hartmut H.-J. Schmidtde, Markus Reiserf, Oliver Marschalg, Martin Steffenh, Michael P. Mannsa, Heiner Wedemeyera
a Abt. Gastroenterologie, Hepatologie und Endokrinologie, Medizinische Hochschule Hannover, Hannover, Germany
b Klinik fur Innere Medizin II, Universitat des Saarlandes, Homburg, Saar, Germany
c Medizinische Klinik II, Krankenhaus Bad Oeynhausen, Bad Oeynhausen, Germany
d Medizinische Klinik m. S. Hepatologie und Gastroenterologie, Charite-Campus Mitte, Berlin, Germany
e Transplantationshepatologie, Universitatsklinikum Munster, Munster, Germany
f Ruhr-Universitat Bochum, Abt. Gastroenterologie und Hepatologie,
Berufsgenossenschaftliche Kliniken Bergmannsheil, Bochum, Germany
g Praxis Braunschweig, Braunschweig, Germany
h Medizinischen Klinik I, Franziskus Hospital Bielefeld, Bielefeld, Germany
ABSTRACT
Therapeutic options for hepatitis C non-responder patients are limited.
Methods: We initiated an open-label pilot study to investigate the efficacy of CIFN plus ribavirin on viral kinetics, sustained virological response (SVR), and histological response in hepatitis C non-responder patients. Seventy-seven patients were enrolled to receive CIFN given daily in combination with 1000/1200mg ribavirin. An 8-week induction-dosing regimen of 18μg CIFN, followed by 9μg for 40 weeks was compared to 9μg CIFN for 48 weeks. 90% of patients were infected with HCV-genotype 1.
Results: Overall, 82% of the patients demonstrated an early virological response, 65% had an end-of-treatment response, and the SVR was 30%. Interferon/ribavirin non-responders demonstrated a SVR of 22%. Induction-dosing resulted in a greater first-phase HCV-RNA decay that, however, did not translate to better SVRs, presumably due to more dose modifications. High ALT, younger age, and second-phase viral kinetics were associated with SVR. Only sustained responders and relapse patients showed an improved liver histology.
Conclusions: Daily dosing of CIFN plus ribavirin may be a promising concept for selected non-responder patients before considering therapies which are anti-viral but not curative. However, motivation and compliance are requisites and a CIFN induction is not required.
".....In conclusion, before starting long-term maintenance therapies with IFN in all non-responder patients, one may consider alternative therapies such as daily dosing of CIFN plus ribavirin to achieve viral clearance. Selected, highly motivated and compliant patients may benefit from this type of therapy....."
1. Introduction
Despite the enormous advances that have been achieved in the treatment of chronic hepatitis C over the last decade [1], there is still a need for improved therapies, especially for the difficult-to-treat patients such as HCV-genotype 1 infected individuals, patients with liver cirrhosis, or patients who did not respond to a previous interferon alfa (IFN)-based therapy [2]. Even the new standard therapy of pegylated interferon alfa (PEG-IFN) in combination with ribavirin is not very effective for the so called non-responder patients. Relapse patients may benefit from re-treatment but patients infected with HCV-genotype 1 who were true non-responders to IFN and ribavirin demonstrated only 12% sustained virological response (SVR) with PEG-IFN and ribavirin [3,4]. Thus, there is currently no generally accepted therapeutic strategy for this patient population. One approach, currently evaluated in clinical trials, is the long-term maintenance therapy with PEG-IFN in non-responders with advanced fibrosis or cirrhosis to prevent hepatocellular carcinoma and/or decompensation (EPIC3, HALT-C, COPILOT trials [3-5]). However, viral eradication should be still the first achievable goal whenever possible. Some study results suggest that consensus interferon (CIFN), which is a 'consensus' molecule of the type-1 interferons with a higher biological activity in vitro [6,7], may be more effective than standard IFN for the difficult-to-treat HCV-genotype 1 patients [8-10]. CIFN monotherapy demonstrated a substantial SVR in non-responder patients [11]. To mimic the half-life of PEG-IFN, CIFN has to be administered on a daily basis. Recently, one study showed that daily dosing of 9μg CIFN significantly increased the SVR compared to a 9μg tiw regimen [12]. Preliminary data from a single center study presented in abstract form, suggested that daily dosing of CIFN in combination with ribavirin can achieve SVRs of 38-45% in non-responder patients to standard IFN and ribavirin depending on the CIFN dose [13]. High-dose-induction, although not effective in studies with standard IFN [14-16], seemed to have further improved the SVR in this study [13]. Cotler and colleagues [17] assessed the first-phase viral kinetics in 20 previous non-responders after a single dose of 15 or 30μg CIFN and demonstrated a significantly sharper decline of the HCV-RNA with the higher dose after 24h (0.8 and 1.5, respectively). Whether high-dose-induction with CIFN may serve as an option to further increase the SVR in difficult-to-treat patients remains unknown.
Here, we initiated an open-label pilot study to assess the efficacy of daily dosing of CIFN in combination with ribavirin on the first- and second-phase of viral kinetics, sustained virological response, and histological response in patients with chronic hepatitis C who did not respond to a prior IFN based therapy. Additionally, we tested if a short-term induction with a double dose of CIFN would further enhance the SVR.
2. Methods
2.1. Selection of patients
Adult patients (18-65 years) with chronic hepatitis C infection were eligible for the study if they were virological non-responders to a previous therapy with IFN or IFN plus ribavirin (HCV-RNA positive after at least 3 MU IFN alfa tiw for 24 weeks). Non-response was further classified as flat non-response (<1log10 reduction of HCV-RNA), flat partial response (>1log10<2log10 reduction of HCV-RNA), and partial response with a significant reduction of viral load (>2log10 reduction of HCV-RNA). However, in a number of patients quantitative HCV-RNA levels of the prior therapy were not available, as this was not standard during former therapies. All patients were seropositive for HCV-RNA by testing with polymerase chain reaction by Roche Amplicor and had elevated serum alanine aminotransferase activity (ALT). Patients were excluded if they had decompensated liver disease, liver diseases unrelated to HCV infection, anemia (hemoglobin concentration less than 12g/dl for women and less than 13g/dl for men), leukocytopenia (less than 3000/μl), thrombocytopenia (less than 100,000/μl), decompensated renal disease (serum creatinine above 130mmol/l), decompensated thyroid disease, HIV or hepatitis B infection, psychiatric conditions, history of seizures, poorly controlled autoimmune diseases, previous organ transplantation. Ongoing intravenous drug (IVDA) abuse and lack of abstinence for at least 12 months were also exclusion criteria. There were two protocol deviations: two patients were included despite platelets lower than 100,000/μl and one of them was also 70 years old. Both patients did not respond to therapy (one breakthrough, one non-response).
3. Study design
The study was an open-label trial with a central randomization procedure performed in Hannover. Seventy-seven patients were randomly assigned into two groups without further stratification using sequentially numbered cards in sealed envelopes. Patients in Group A were treated with 9μg CIFN daily for 48 weeks whereas patients in Group B received an induction therapy of 18μg CIFN daily for the first 8 weeks followed by 9μg CIFN daily for the remaining 40 weeks. In both groups, ribavirin was given at the standard dose of 1g (<75kg) or 1.2g daily (>75kg).
The patients were evaluated as outpatients at the following visits: screening visit, entry visit, day 1, day 3, week 1, 2, 4, 12, 18, 24, 30, 36, 42, 48, 52, 60, and 72. The laboratory of each center performed biochemical and hematological testing. Serum HCV-RNA levels (IU/ml) were determined with the Cobas Amplicor Hepatitis C Monitor Test (v2.0, Roche Diagnostics). Viral genotypes were determined with the INNO-LiPA HCV II Kit (Innogenetics, Gent, Belgium).
This protocol was approved by the local ethics committees, and was conducted according to principles of the Helsinki Declaration. Written informed consent was obtained from all participants prior to enrollment.
4. Assessment of efficacy
The primary endpoint was sustained virological response (SVR), defined as undetectable HCV-RNA in serum 24 weeks after the end-of-treatment (EOT). Secondary endpoints were early virological response (EVR: HCV-RNA decline>2log10 before week 12) and absence of serum HCV-RNA at the EOT and normalization of serum ALT at the end of follow-up. Therapy was discontinued once the HCV-RNA was detectable at week 24 of treatment. The first-phase of early viral kinetics was assessed by the log10 decay during the first 24h as by Cotler et al. [17]. The second-phase slope of the initial viral kinetics was calculated by log-linear regression on measurements of HCV-RNA at 1, 2, and 4 weeks and rapid virological response (RVR) was defined as a second-phase slope faster than -0.10/day as previously [18]. In 37 patients, a second liver biopsy was performed at the end of the follow-up (24 weeks after end of therapy) and the histology (Ishak score [19]) was compared to the histology before the initiation of therapy.
5. Statistical analysis
Data were described by rates, means with standard deviation, medians, and ranges. Furthermore, groups were compared by X2 test, Fishers exact test, Man-Whitney and Kruskal-Wallis test as appropriate. In addition, multivariate step-wise logistic regression was used to identify independent predictors from baseline characteristics which were associated with SVR in univariate analysis. All p values reported were two-sided and p values below 5% were considered significant.
6. Results
6.1. Patients
Between March 2000 and September 2001, 77 patients were randomized (Group A=38, Group B=39 and treated at eight different sites (Hannover, Bad Oeynhausen, Berlin, Bochum, Braunschweig, Bielefeld, Kassel, Koblenz). The baseline characteristics are shown in Table 1. The only significant difference between the treatment groups was found in baseline ALT as Group B tended to have lower ALT levels (p=0.048).
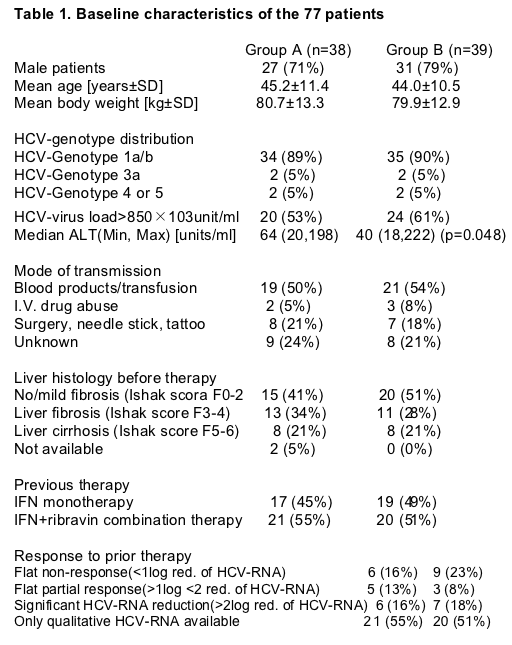
6.2. Safety
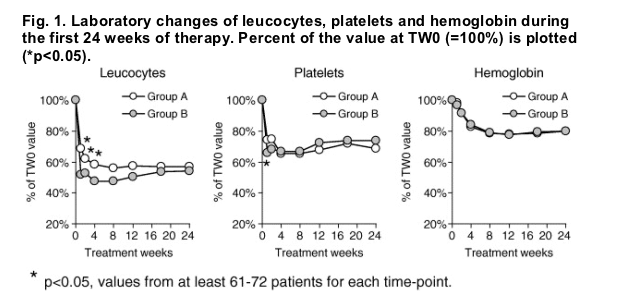
The spectrum of side effects of daily CIFN therapy was comparable to previous trials with IFN and ribavirin combination therapy. However, the high-dose-induction-phase had a high side effect profile. There were four serious adverse events (suspected drug related) during therapy (Table 2). A case of menigococcus sepsis occurred during the last 2 weeks of therapy in Group A. Despite discontinuation of therapy, the patient remained HCV-RNA negative. All other patients with serious adverse events discontinued treatment and relapsed or remained HCV-RNA positive. There was a trend towards more severe flu-like symptoms and nausea in the induction group, which resulted in more frequent dose modifications early during treatment. Reduction of leucocytes and platelets were significantly higher in the induction group during the first 8 weeks of therapy (leucocytes TW1, TW2, TW4; platelets TW1, Fig. 1). Due to laboratory abnormalities and the side effects, almost every other patient required a reduction of the 18μg CIFN dose before week 8, while this was the case in only 13% of patients receiving 9μg CIFN during the first 8 weeks (p=0.001, Table 2). Patients in Group A developed anemia and associated side effects (dyspnoe, vertigo) more frequently than patients in Group B, resulting in ribavirin dose modifications in twice as many patients in Group A than B (p=0.04). However, the overall percent reduction of the hemoglobin was not different between both groups. The reason for this finding may have been the lower hemoglobin concentration in Group A at TW0, which was almost significant. No signs of diminished liver function (as measured clinically and by coagulation function and serum albumin) were noted during therapy. We had no evidence for hypocalcemia as previously reported for daily dosing of CIFN [20].
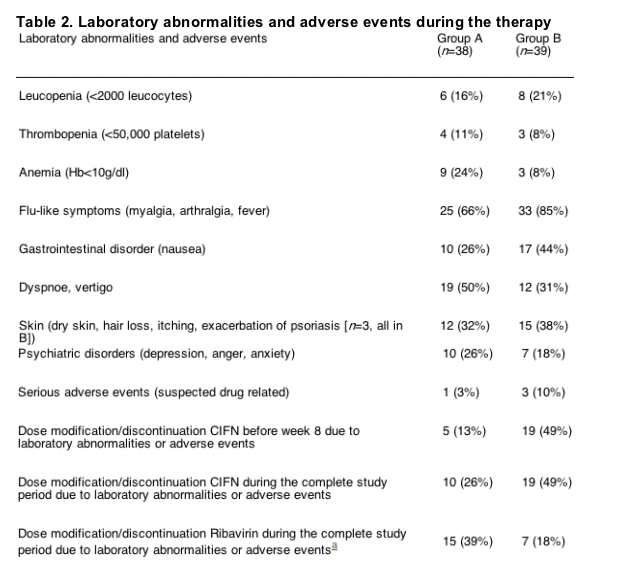
The noted adverse events were moderate to severe (subjective reports from patients) and occurred at least once during the treatment period. Serious adverse events: (A) meningococcus sepsis; (B) severe weight loss and poor physical condition resulted in drop out, two patients with injection site abscesses resulting in surgical procedures.
aRibavirin dose reductions did not significantly affect the relapse rate in this study.
6.3. Efficacy
Overall, 59 patients (77%) completed more than 42 weeks of therapy (>80% of treatment duration), whereas 4 patients dropped out before TW24. In 12 patients, therapy was stopped at TW24 due to non-response, and 2 patients discontinued the study between TW24 and TW42 (Fig. 2). 50 patients (65%) had an EOT response. There was no difference between the two treatment groups (Fig. 3A). Unfortunately, 26 of the EOT patients (52%) had a virological relapse during the follow-up period. Twenty-three patients (30%) had a SVR and one patient was lost to follow-up (Fig. 2). Again, there was no difference between both treatment regimens (Fig. 3A).
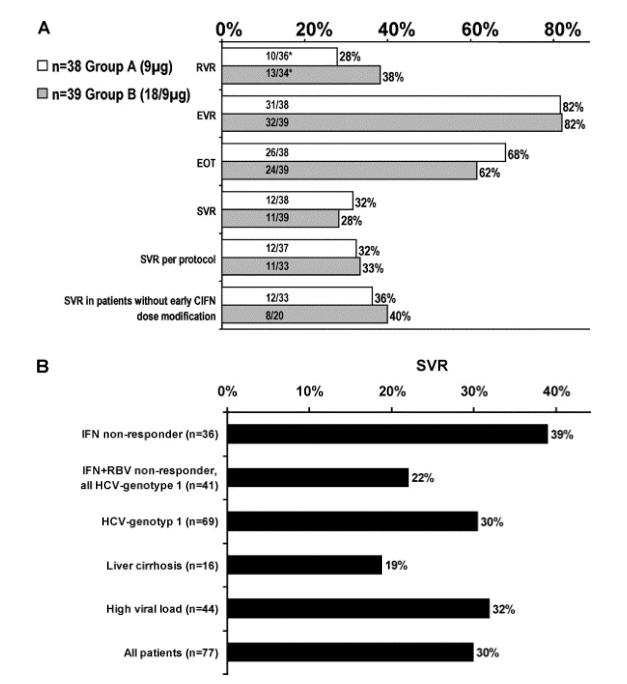
Fig. 3. (A) Rapid virological response (RVR: second-phase slope faster than -0.1/day), early virological response (EVR:>2log10 decline of HCV-RNA before week 12), end of treatment response, sustained virological response, sustained virological response in patients who were treated according to the protocol, and sustained virological response rates of patients without CIFN modifications in the first 8 weeks are shown. (B) Sutained virological response rates of patients with different baseline characteristics are shown. Non-responders to IFN monotherapy had the best sustained response and patients with liver cirrhosis had the poorest sustained response rates.
Comparing the HCV-RNA levels during therapy, we observed a more pronounced decline in HCV-RNA in patients treated with 18μg CIFN during the first 8 weeks of therapy. Especially the median log10 drop of HCV-RNA after 24 and 72h was 0.29 and 0.57 in Group A, and 0.30 and 1.03 in Group B (p=0.16 and 0.002, respectively). The stronger first-phase decline in HCV-RNA resulted indeed in a higher virological response at TW8, as 56% of patients treated with the high dose were already HCV-RNA negative compared to only 42% in Group A (Fig. 4A). However, the number of patients with an EVR defined as>2log10 reduction at TW12 was not different between both groups (Fig. 3A).
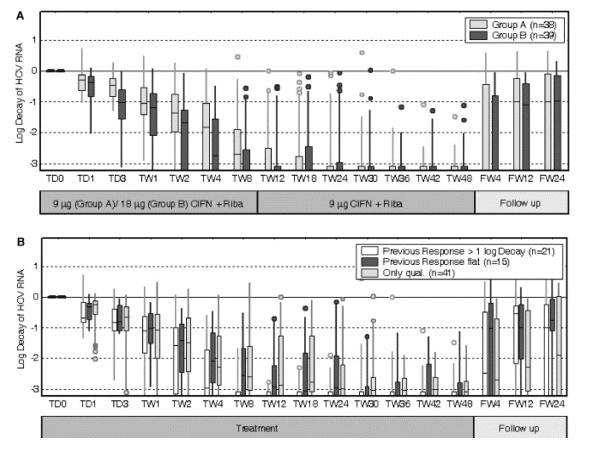
The RVR as defined by Layden [18], that is a second-phase slope during TW1 and TW4 faster than -0.1/day, was not statistically different between the treatment groups (28% in Group A, 38% in Group B, p>0.2).
6.4. Factors influencing sustained virological response
It is known that patients who are adherent to therapy respond much better to therapy than those who are not compliant [21]. Here, patients who were treated according to the protocol and did not drop out showed a SVR of 33% (Fig. 3A). The SVR was even higher in the group of patients that did not require CIFN dose modifications during the induction phase.
When comparing baseline characteristics in patients with and without SVR, only high baseline ALT and low age were significantly associated in univariate analysis (Table 3) and both variables remained significant in multivariate logistic regression (p=0.001, 0.046, respectively). Interestingly, SVR was significantly but not exclusively associated with RVR as 13 of 23 (57%) patients with RVR but also still 10 of 47 patients (21%) without RVR achieved a SVR (Table 3).
Nevertheless, patients who were non-responder to an IFN monotherapy showed a higher chance to achieve a SVR (39%) than IFN/ribavirin non-responder (22%, p=0.1, Fig. 3B). This trend was also seen for patients with a faster HCV-RNA decay during the previous therapy as the median log10 decay after 8 weeks of treatment was 3.38 in patients with a prior HCV-RNA decay>1log10 compared to 2.35 in patients with a flat response during the previous therapy (p=0.07, Fig. 4B).
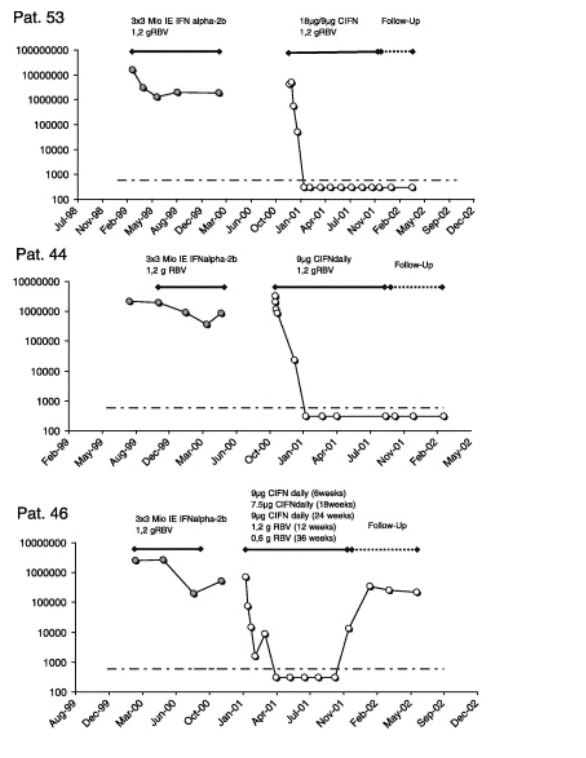
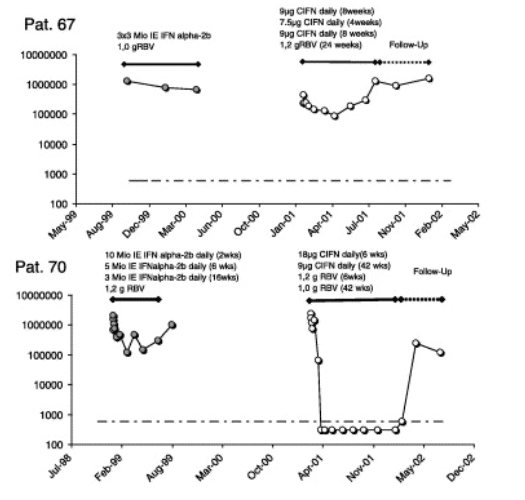
For six patients we were able to compare the HCV-RNA kinetics from the prior therapy to the current CIFN treatment (Fig. 5). All six patients were previously treated in well-documented studies and were compliant according to the patient records. Interestingly, different patterns of HCV-RNA kinetics during earlier and current treatment were observed. Three out of four patients who were treated with 3 MU IFN alfa-2b tiw during the prior therapy had a rapid viral decline of HCV-RNA and two of these had received induction dosing of IFN alfa-2b during their prior treatment. Both were able to clear HCV-RNA during treatment with CIFN. However, this was not true for patient 67 who seemed to be resistant also to CIFN therapy (Fig. 5).
6.5. Histological response
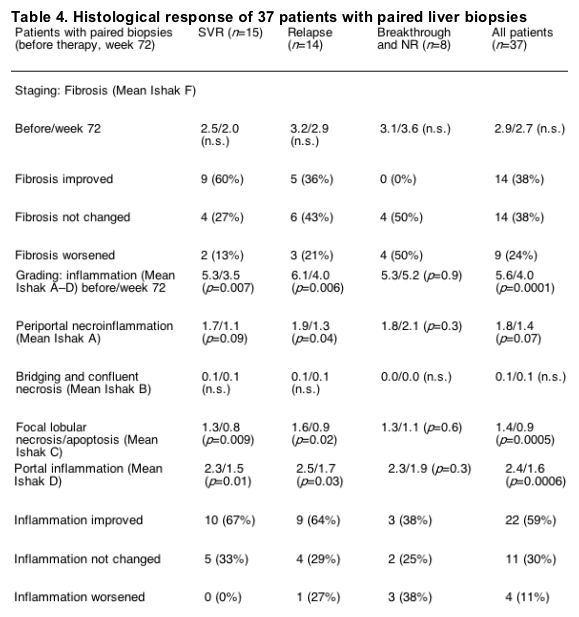
All patients underwent liver biopsy before the start of therapy. Thirty-seven patients had a second liver biopsy at the end of the follow-up. Patients with SVR had the best histological response, and relapse patients had a better response than did non-responders or breakthrough patients (Table 4). Only three of the non-responder patients had an improvement in the grading but none of them improved in the fibrosis score. In contrast, 60% of the patients with a SVR improved their fibrosis stage.
7. Discussion
Currently, there are no recommendations how to manage patients with chronic hepatitis C who did not respond to a prior IFN/ribavirin therapy. Here, we investigated a daily dosing regimen with CIFN in combination with ribavirin. As expected for non-responders, our 77 patients showed baseline characteristics that are associated with a poor treatment response (Table 1). The treatment with daily dosing of CIFN plus ribavirin resulted in surprisingly high EVR of more than 80% but after the end of therapy a high relapse rate was observed. The overall SVR was 30% for this difficult-to-treat patient group. The most important group of patients in our study population, the IFN/ribavirin non-responder group achieved a SVR of 22%, which is about 5-10% higher than reported in prior non-responder studies [3,4,22].
However, it is always a problem to compare studies, especially non-responder trials. Non-responder patients are a highly heterogeneous group (Table 1). It is sometimes difficult to determine if the patients were true non-responders to the prior therapy or if these patients were just non-compliant. Another factor contributing to the heterogeneity of the non-responders is the response kinetic during the previous therapy. The different response kinetics during prior therapies may influence the response during the re-treatment. Patients who achieved a >1log10 decay of HCV-RNA in the earlier therapy had a sharper viral decline during the re-treatment (Fig. 4B). One limitation in this study and in many other studies is that the response kinetic to the prior therapy is unknown in many patients (Table 1). Comparing the response to the prior therapy with the response to the daily CIFN/ribavirin treatment in individual patients, it seems that CIFN can indeed have a higher antiviral potency in some patients (Fig. 5). The high EVR in our study but on the other hand the high relapse rate may raise new questions and ideas for further studies. A longer treatment with CIFN plus ribavirin might be an option for these patients.
In this specific difficult-to-treat patient group, age and baseline ALT were independent predictors of SVR whereas other well-known predictive factors for SVR as, e.g. HCV-genotype, were not significantly associated with SVR here (Table 3). In Layden et al. [18], RVR defined as a second-phase slope during TW1 and TW4 faster than -0.1/day was highly predictive for SVR in previously untreated patients receiving CIFN monotherapy. They reported that no patient without RVR achieved SVR. Here, RVR remained to be associated with SVR but even 23% of the patients without RVR achieved SVR. This may be due to combination therapy with ribavirin, mainly.
Adherence to therapy is another important factor for the success of the treatment [21]. Therefore, therapies that induce severe side effects are in the end less effective despite higher antiviral efficacy. The adherence to therapy here was comparable to previous tiw CIFN regimens [10]. However, in contrast to a report from the USA in treatment naive patients [20], 9μg daily CIFN in combination with ribavirin were well tolerated in our study. The selection of non-responders in our study who are motivated and already tolerated a prior IFN therapy may account for this discrepancy. Another German study also reported good tolerability of a daily 9μg CIFN plus ribavirin regimen in non-responders (P. Buggisch personal communication). However, the induction-dosing of 18μg CIFN in combination with ribavirin required early dose modification due to side effects and laboratory abnormalities in 49% of the patients such as patient 32 and 70 (Fig. 5). This is in line with a previous non-responder study from Canada [23]. Three of our patients treated with the high dose even dropped out due to adverse events before TW8. This might help to explain why the overall response rates were not higher in Group B (Fig. 3A) despite the initially higher response rates (Fig. 4A). The stronger viral decline in the first-phase with the induction-dosing might reflect the higher antiviral efficacy of a higher dose but due to dose reductions and the comparable RVR, this effect could not be transferred into SVR. Layden et al. discussed another possibility that might help to explain why an induction regimen is in the end less effective as they speculate that induction would select for a therapy resistant virus strain [18]. Overall, our data suggest that the high-daily-dose-induction is not superior.
The patients with liver cirrhosis had as expected very low response rates. Patient 67 is an example with almost no response to therapy (Fig. 5). This male patient had HCV-genotype-1, was older than 45 years, had relative low baseline ALT levels, had liver cirrhosis, and had a flat non-response to the prior IFN/ribavirin therapy. Furthermore, a dose modification of CIFN was required due to thrombopenia. Unfortunately, these patients who would benefit the most from a curative antiviral treatment have the worst outcome. These patients may be better treated with low dose interferon maintenance therapy to prevent complications of liver cirrhosis, although only one study to date has demonstrated any benefit in this approach [5].
We were also interested in the effect of CIFN on the histology as we compared the liver biopsies from 37 patients before and after therapy (Table 4). As expected, the patients who demonstrated a SVR had the best improvement in staging and grading (Table 4). Only 2 of the 15 sustained responder analyzed had a worsening in the fibrosis score. Both patients had elevated transaminases at the end of follow-up and high gamma-GT values, possibly due to alcohol consumption. Also the relapse patients showed a histological benefit after the end of follow-up (Table 4). In contrast, patients with only short-term virus suppression (breakthrough) or a complete non-response demonstrated no histological benefit. This data suggest that only a long-term virus suppression or clearance can lead to a histological response.
In conclusion, before starting long-term maintenance therapies with IFN in all non-responder patients, one may consider alternative therapies such as daily dosing of CIFN plus ribavirin to achieve viral clearance. Selected, highly motivated and compliant patients may benefit from this type of therapy.
|
|
| |
| |
|
|
|