| |
Hepatitis/HIV in Europe: 10% of HCV+ initiated therapy
|
| |
| |
Therapeutic management of hepatitis and HIV infection in co-infected patients: Results of a survey performed before the 2005 Consensus Conference
....Overall, 10% of the patients had initiated an anti-HCV treatment at the end of 2004.....survey, performed before the First European Consensus Conference on the Treatment of Chronic Hepatitis B and C in HIV Co-infected Patients.... The high frequency of adverse events reported during anti-HCV therapy, and particularly of neuropsychological side effects, is probably another limitation to treatment......The problem is slightly different for HBV co-infection. Lamivudine has been available for many years and mainly given to HBsAg positive patients. This widespread use has given a false assurance and, as a result, most chronic HBV disease is not well evaluated or treated. The recent availability of adefovir and tenofovir facilitates dual therapy for chronically infected HBV/HIV co-infected patients and should prevent the emergence of resistance....."
Journal of Hepatology
Volume 44, Issue (Supplement 1), Pages S2-S5 (2006)
Dominique Salmon1, Mathieu Robain2, Jurgen K. Rockstroh3, Yves Benhamou4
1Infectious Diseases Unit, Cochin Hospital, Rene Descartes University (Paris V), 27, rue du Faubourg Saint Jacques, 75014 Paris, France
23Es company, Paris, France
3Department of Medicine I, University of Bonn, Germany
4Hopital Pitie-Salpetriere, Paris, France
The First European Consensus Conference on the Treatment of Chronic Hepatitis B and C in HIV Co-infected Patients aimed to produce recommendations that could be applied across Europe. However, some important differences exist around Europe, in terms of access to treatment and tests for monitoring.
This short survey of 24 European countries showed that access to anti-HCV treatment is low (approximately 10%) in patients with HCV/HIV co-infection-generally access is higher in Western Europe than in Eastern or Northern Europe.
This low use of anti-HCV therapy is not a result of poor availability (of drugs or virological tests), which are available in all countries surveyed.
Recently published trials in co-infected patients and the outcome from this Consensus Conference will hopefully lead to wider access to anti-HCV therapy and better management of co-infected patients across Europe.
Article Outline
- Abstract
- 1. Introduction
- 2. Methods
- 3. Results
- 3.1. Participation and responses rates
- 3.2. Access and reimbursement to anti-viral drugs
- 3.2.1. Anti-HIV drugs
- 3.2.2. Anti-HCV drugs
- 3.3. Availability and reimbursement of virological tests
- 3.4. Liver fibrosis evaluation
- 3.5. Access to anti-HCV treatment
- 4. Discussion
1. Introduction
In Europe, the prevalence of hepatitis C virus (HCV) infection in HIV-infected patients is particularly high compared to the rest of the world, and the care of patients harbouring both viruses is a major challenge for the future. A recent EuroSIDA survey showed that 33% of HIV-infected patients were HCV co-infected, with a lower prevalence in Northern and Central Europe (23.2 and 20.4%, respectively) than in Southern and Eastern Europe (41.4 and 46.9%, respectively) [1]. Conversely, hepatitis B virus (HBV) and HIV co-infection is less frequent in Europe than in other regions of the world, such as Asia or Africa, but the hepatic problems posed by HBV are similar to those posed by HCV. Moreover, several anti-HIV drugs are concomitantly active on HBV and, therefore, care is needed to treat both viruses at the same time while avoiding the emergence of resistance. End-stage liver disease is now the predominant cause of death in patients co-infected by HCV and is also a leading cause in HBV infection [2] despite the availability of treatments with a proven efficacy. Most patients are not treated-underlying the need for treatment guidelines.
Because European countries share a common agency for drug approval (EMEA) and are recognized as a single entity at international level, we decided that a European Consensus Conference on the treatment of hepatitis in HIV-infected patients was due. Although important differences in epidemiology and access to care are present between Western and Eastern Europe, the mission of the Jury of this Consensus Conference was to build recommendations that are applicable to the majority of European countries, even if it was clear that these recommendations would need to be adapted locally.
It therefore became important for us to gain a better knowledge of the accessibility to anti-HIV and anti-hepatitis treatment in different European countries. This was the rationale behind the short survey that we conducted before the Consensus Conference, and which we describe here.
2. Methods
A questionnaire to evaluate the ease of access to anti-HIV and anti-hepatitis treatment in the different European countries was developed. It contained closed questions concerning the access and reimbursement of anti-HIV and anti-hepatitis drugs, their availability in public and/or private structures, the availability of virological tests and their modalities of use, how and when hepatitis therapy is initiated, the need or not of liver fibrosis evaluation before therapy, and open questions on the percentage of treated patients and the difficulties encountered. The feasibility of the questionnaire was tested in two countries before sending it to all European countries via three channels:
- The local WHO correspondent of each country.
- Patient advocates of the European Association Treatment Group (EATG).
- The local correspondent of biomedical industries.
The questionnaire was sent in December 2004 and responses were received between December 2004 and February 2005.
3. Results
3.1. Participation and responses rates
The questionnaire was sent to the 25 countries of political Europe, one additional country responded (26 in total) and evaluative responses were received from the following 23 countries: Austria, Bulgaria, Czech Republic, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Malta, Netherlands, Poland, Portugal, Serbia, Spain, Sweden, Turkey, Ukraine, Switzerland, United Kingdom, and Finland. The number of responses from each country ranged from 1 to 3.
3.2. Access and reimbursement to anti-viral drugs
3.2.1. Anti-HIV drugs
Nearly all drugs commercially labelled in Europe were available without restriction, with only a few exceptions (for example, atazanavir was restricted to pre-treated patients in some countries and T20 is restricted in all countries), and in 21 out of 23 countries, anti-HIV drugs were free of charge for the patient (Figs. 1 and 2).
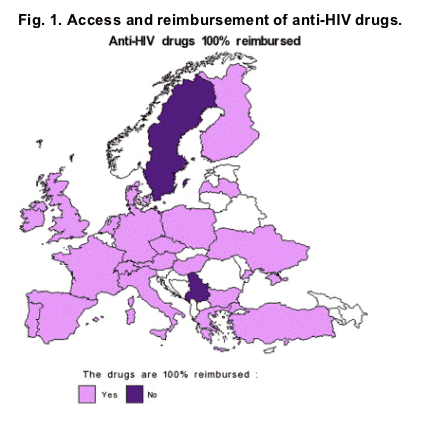
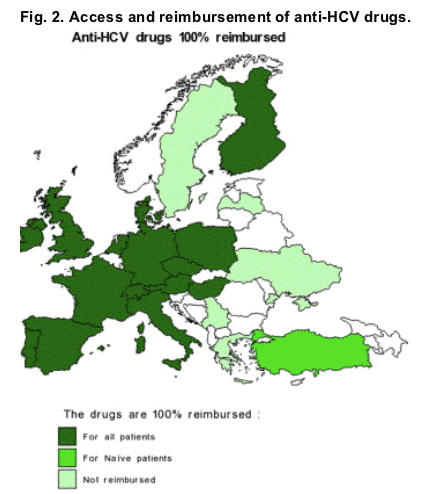
3.2.2. Anti-HCV drugs
Anti-HCV drugs that are the standard of care (i.e. peginterferon and ribavirin) were available in all countries. However, they were not supplied by public hospitals in 14% (3/22) of the countries and/or by private pharmacies in 33% (7/21) of the countries.
Anti-HCV drugs were not free of charge in all European countries and the reimbursement depended on the patient type-the reimbursement rate varied from 73% for naive patients to 68% for relapsers/non-responders and to 55% for patients with acute hepatitis.
3.3. Availability and reimbursement of virological tests
Qualitative HCV-RNA and quantitative HBV-DNA testing were available in all countries participating in the survey.
Qualitative HCV-RNA testing was available only in public hospitals in 38% of the countries (8/21). The test was reimbursed to the patient in 91% of countries (21/23). The number of tests per year for any single patient was limited in 17% of the countries (3/18 responders)-to two tests/year in two countries and three tests/year in the others.
Quantitative HBV-DNA testing was available only in public hospitals in 33% of the countries (7/21). The test was reimbursed to the patient in 91% (21/23) of countries. The number of tests per year for a single patient was limited in one country to two tests/year.
3.4. Liver fibrosis evaluation
Liver fibrosis evaluation was required before anti-HCV treatment was initiated in 50% of the countries participating in the survey (11/22 responders). In eight of these 11 countries, this requirement was extended to patients with any HCV genotype, and for three countries it was limited to patients with HCV genotype 1 (Fig. 3).
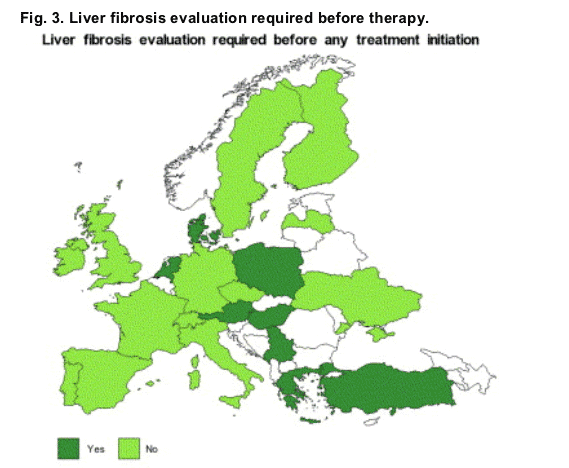
3.5. Access to anti-HCV treatment
The percentage of HIV/HCV co-infected patients treated for HCV was low and varied from 0 to 23%. Overall, 10% of the patients had initiated an anti-HCV treatment at the end of 2004. Access to treatment was higher in Western Europe than in Eastern or Northern Europe (Fig. 4).
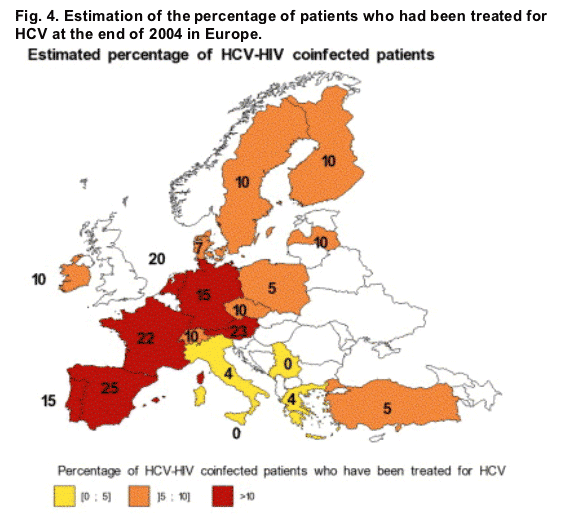
Initiation of anti-HCV treatment was performed only in public hospitals in 55% of the countries (11/20) but anti-HCV treatment was not generally initiated by one specific specialist. The initiation only by hepatologists of anti-HCV treatment was possible in 29% (6/21) countries.
The number of anti-HCV treatments for any given patient was restricted in 41% of the countries (9/22).
4. Discussion
This survey, performed before the First European Consensus Conference on the Treatment of Chronic Hepatitis B and C in HIV Co-infected Patients, showed that a very low percentage of co-infected patients have been treated for their hepatitis, despite drugs and virological tests being available in most countries.
This is understandable because until 2004 the evidence for activity of anti-HCV drugs had been demonstrated only in HCV mono-infected patients. However, the recent publication of four pivotal studies has made it clear that the optimal treatment for chronic hepatitis C in HIV-infected persons is peginterferon and ribavirin [3-6]. Other factors may also explain the poor access to care. For example, in Europe the main risk factor for HCV is drug use and a great number of these patients continue active drug use and do not have access to substitution. An improvement of global care, including anti-HIV treatment and anti-HCV treatment, would probably be achieved by offering greater access to substitution therapy to active drug users.
Another factor that may discourage anti-HCV treatment initiation is the lack of guidelines for diagnosis, evaluation of the severity of HCV disease and treatment.
The high frequency of adverse events reported during anti-HCV therapy, and particularly of neuropsychological side effects, is probably another limitation to treatment.
The problem is slightly different for HBV co-infection. Lamivudine has been available for many years and mainly given to HBsAg positive patients. This widespread use has given a false assurance and, as a result, most chronic HBV disease is not well evaluated or treated. The recent availability of adefovir and tenofovir facilitates dual therapy for chronically infected HBV/HIV co-infected patients and should prevent the emergence of resistance.
In conclusion, this survey has underlined the fact that the recommendations elicited by the Consensus Conference Jury should be very useful for improving the global care of patients co-infected by HIV and hepatitis, thus preventing the evolution towards end-stage liver disease.
References
1. [1]Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, et al.. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992-1002.
2. [2]Salmon-Ceron D, Lewden C, Morlat P, Bevilacqua S, Jougla E, Bonnet F, et al.. Liver disease as a major cause of death among HIV-infected patients: roles of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799-805.
3. [3]Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, et al.. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. J Am Med Assoc. 2004;292:2839-2848.
4. [4]Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, et al.. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438-450.
5. [5]Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al.. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-co-infected persons. N Engl J Med. 2004;351:451-459.
6. [6]Laguno M, Murillas J, Blanco JL, Martinez E, Miquel R, Sanchez-Tapias JM, et al.. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27-F36.
|
|
| |
| |
|
|
|