| |
Review article: predicting response in hepatitis C virus therapy
|
| |
| |
".....Despite major improvements in anti-HCV therapy within the last years, treatment of chronic hepatitis C is still challenging and further optimization is needed. Many patient- and viral-related factors have been evaluated for an association with virologic response to interferon-alpha-based antiviral therapy. Patient-related factors have so far not lead to individualized treatment recommendations...." Note from Jules Levin: This review is a nice update on the various factors and approaches, also called algorithms, that affect predicting response to peginterferon plus ribavirin therapy.
U. MIHM, E. HERRMANN, C. SARRAZIN AND S. ZEUZEM
Department of Internal Medicine II, Saarland University, Homburg/Saar, Germany
Alimentary Pharmacology & Therapeutics
Volume 23 Page 1043 - April 2006
Summary
The introduction of combination therapy with ribavirin and of pegylated interferons has improved treatment results in patients with chronic hepatitis C. However, overall rates of sustained virologic response following antiviral therapy of chronic hepatitis C still do not exceed 54-63%. Because of several virus- and patient-related factors, treatment is even less successful in some patient subpopulations.
The major viral factors associated with impaired response are hepatitis C virus genotype 1 infection and a high viral load. Among patient-related factors cirrhosis is of special importance. Baseline predictive factors for sustained virologic response become less important for prediction of treatment outcome when quantifications of hepatitis C virus RNA during early therapy are taken into account.
This article provides a summary of virus- and patient-related parameters, which are prognostic for response to antiviral therapy in chronic hepatitis C and focuses on the prediction of treatment response by quantification of hepatitis C virus RNA concentration during therapy.
Introduction
Chronic infection with the hepatitis C virus (HCV) is an important cause of liver cirrhosis and its complications like ascites, haemorrhage from oesophageal and gastric varices and hepatocellular carcinoma.13 Eradication of HCV by antiviral treatment improves liver histology and patient survival.4, 5 Therefore, the primary goal of antiviral therapy in chronic hepatitis C is sustained virologic response, defined as undetectable serum HCV RNA by a sensitive molecular assay 24 weeks after the end of therapy. However, by current standard therapy, comprising 24-48 weeks of treatment with pegylated interferon-alpha and ribavirin, rates of sustained virologic response to treatment are still unsatisfactory with only about 54-63%.68
Identifying factors for virologic outcome to antiviral therapy may allow individualized modifications of treatment regimens, which subsequently could improve treatment response. As well, treatment with pegylated interferon (peginterferon)-alpha and ribavirin is associated with many side effects and early detection of non-sustained virologic responders is of major importance to avoid unnecessary treatment-related morbidity in these patients.
In recent years, several viral- and patient-related factors which affect response to interferon-alpha-based antiviral therapy have been identified. Interestingly, baseline predictive factors for sustained virologic response become less important for prediction of treatment response when quantifications of HCV RNA during early therapy are applied.9-12 The present review gives an overview on viral- and patient-related factors associated with different responses to interferon-alpha-based antiviral therapy and discusses viral load assessments during treatment for prediction of virologic therapy success.
Methods
Literature search was performed by screening MEDLINE for consensus statements on the therapy of chronic hepatitis C infection from 2000 to 2005 and peer reviewed English-language articles (1990 through November 2005) by using the following Medical Subject Heading terms and keywords alone and in appropriate combinations: chronic hepatitis C, treatment, response, prediction, interferon, ribavirin, sustained virologic response, relapse, non-response, genotype, viral load, HCV RNA, cirrhosis, ethnicity, age, treatment algorithm, model and viral kinetics. The review is based on prospective and retrospective analyses of large randomized, controlled multicentre trials. All pivotal trials for therapy with standard interferon-alpha plus ribavirin, peginterferon-alpha and peginterferon-alpha plus ribavirin were considered. Results from smaller, non-randomized, open-label studies have been included if these studies were performed with adequate methodology as judged by the authors. Data published only as abstracts have been rejected.
Patient-related factors
One of the most important patient inherent predictors for reduced rates of sustained virologic response is advanced fibrosis or cirrhosis.13, 14 In four retrospective analyses of randomized, controlled multicentre trials a sustained virologic response was observed in 8-44% of patients with septal fibrosis or more advanced disease, with higher response rates for treatment with peginterferon-alpha and ribavirin.6, 8, 13, 15 However, even if HCV eradication was not achieved in roughly one third of non-sustained virologic responders a histological improvement was reported.15
Belonging to the ethnic group of African-Americans is another factor which was found associated with a less favourable response to treatment in a retrospective subgroup analysis of two randomized, controlled multicentre trials16 and in two recent prospective controlled, multicentre trials17, 18 with rates for sustained virologic response ranging from 11% for treatment with standard interferon plus ribavirin16 to 19-26% for therapy with peginterferon plus ribavirin.17, 18 The reasons for this phenomenon are not clear, so far. However, increased iron stores and differences in the immune response compared with White patients have been reported.19-22
Other patient-related factors influencing therapy outcome negatively are male gender, older age, higher body weight or body mass index, liver steatosis and elevated pretreatment serum gamma glutamyltransferase (GGT) levels.69, 13, 14, 23-34 The pathogenetic background of GGT elevation is not yet fully elucidated, however a close correlation between serum GGT levels and hepatic steatosis, fibrosis and intrahepatic expression of tumour necrosis factor-a was described.35-37
VIRAL FACTORS
HCV genotype
The most important baseline predictor for response to antiviral combination therapy with interferon-alpha and ribavirin is HCV genotype. HCV genotype 1 has been shown to be associated with lower rates of sustained virologic response compared with genotypes other than 1 by logistic regression analysis.68, 28, 3032 An HCV genotype other than 1 independently and significantly increases the odds of achieving sustained virologic response with odds ratios of 3.25 and 5.4 in different trials on combination therapy of peginterferon-alpha-2a plus ribavirin.6, 7 Rates of sustained virologic response after treatment with peginterferon-alpha plus ribavirin for 48 weeks range from 41 to 52% in patients with HCV genotype 1 infection (Table 1).68 However, when treated with the same combination therapy regimen for 24 to 48 weeks, patients infected with HCV genotype 2 or 3 achieve sustained virologic response rates of 76-84% (Table 1). 68, 33 Furthermore, it has been shown that a shorter treatment duration of 24 weeks compared with 48 weeks does not impair sustained virologic response rates in patients with HCV genotype 2 or 3 infection.7, 30, 31, 33 In this patient group treatment with peginterferon-alpha-2a or 2b plus ribavirin for 24 weeks results in sustained virologic response rates of 81-84% as compared with sustained virologic response rates of 76-82% for treatment with peginterferon-alpha-2a or 2b plus ribavirin for 48 weeks.68, 33 In patients with HCV genotype 2 or 3 infection, flat doses of 800 mg ribavirin daily in combination with peginterferon-alpha-2a are proven to be sufficient (Table 1).7 For the use of pegylated interferon-alpha-2b, however only data with weight-based dosing are currently available.33
These data lead to different treatment recommendations for patients infected with HCV genotype 1 and patients infected with HCV genotypes 2 or 3 (see below). The reasons for the different virologic response rates in different HCV genotypes are complex and not yet fully understood. Viral kinetic analyses suggest that during interferon-alpha-based therapy, clearance of infected hepatocytes is slower and antiviral efficacy is reduced in patients infected with HCV genotype 1 compared with infections with other HCV genotypes.38, 39
HCV baseline viral load
Whereas baseline viral load does not seem to influence progression of HCV-related liver damage, patients with a higher baseline viral load tend to respond less to interferon-alpha-based antiviral treatment. In the registration trials for combination therapy of standard interferon-alpha plus ribavirin and for monotherapy with peginterferon-alpha-2a and peginterferon-alpha-2b, it was shown by logistic regression analysis that a low viral load (2 X 106 c/mL or less) is an independent predictor for sustained virologic response.28, 3032 Similar observations were made in the registration trial for combination therapy of peginterferon-alpha-2b plus ribavirin. A low baseline viral load (2 X 106 c/mL or less [2 million]) was significantly associated with improved rates of sustained virologic response by multivariate logistic regression (Table 1).8 In this trial, patients who were treated with peginterferon-alpha-2b 1.5 mg/kg/week plus ribavirin 800 mg daily and who had a low baseline viral load achieved a sustained virologic response rate of 78% as compared with 42% in patients with a high pretreatment viral load (Table 1).8 In the corresponding trial with peginterferon-alpha-2a and ribavirin combination therapy, pretreatment viral load was not among the predictors of sustained virologic response that were identified by a multiple logistic regression model. Nevertheless, patients with a low viral load (2 X 106 c/mL or less) showed substantially higher rates for sustained virologic response than patients with a high viral load (62% vs. 53%; Table 1).6
Viral kinetics during therapy
Mathematical modelling of viral kinetics during therapy
Hepatitis C virus RNA decay during antiviral therapy typically shows a biphasic pattern with a rapid first phase during the initial 24-48 h and a less rapid second phase of viral decline thereafter. Viral kinetics on the basis of frequent quantifications of HCV RNA during the first 2-6 weeks can be explained by mathematical models. Standard mathematical compartment models for hepatitis C viral kinetics include compartments of infected and uninfected cells as well as a compartment of free virus in serum.25, 38-50 Moreover, those models assume that the influence of the compartments on each other can be estimated by constant rate factors. The constant rate factors comprise rates for viral production per infected cell, de novo infection of uninfected cells, death or degradation rates of free virus, infected and uninfected cells, production rate of uninfected target cells as well as treatment efficiency factors on viral production or on de novo infection. Importantly, there has not been a single situation yet reported where a slower viral decay was associated with an improved sustained virologic response to anti-HCV therapy. Thus, viral kinetic analysis can be used as an early predictive marker for sustained virologic response. Although first-phase viral kinetic parameters are associated with sustained virologic response,40, 43, 44, 51-54 an even stronger association has been observed between second-phase viral kinetic parameters, especially the rate of infected cell loss, and sustained virologic response.39, 40, 42, 44, 49, 52-54
Despite these obvious associations, obtaining mathematically modelled viral kinetics cannot be performed for the majority of patients treated outside clinical trials, and thus is not feasible for management of antiviral therapy in individual patients in clinical practice.
Treatment algorithms based on HCV RNA decay at specific time points during therapy
Several early virologic response criteria have been analysed for prediction of virologic response to an interferon-alpha-based antiviral treatment. Most investigations aimed at defining algorithms for early detection of non-responders and early treatment discontinuation in these patients.6, 9-12, 55, 56 Recently, however, some clinical trials used early HCV RNA decay during therapy to identify patients with rapid response in whom treatment might be shortened compared with the standard therapy without compromising sustained virologic response rates.5760 In one clinical trial, individualization of therapy - including shortened or prolonged treatment as well as addition of histamine or application of different dosing of peginterferon-alpha - according to early viral decline was examined but did not lead to an improvement of overall virologic response rates compared with standard therapy.61
Treatment algorithms for early discontinuation of non-responders
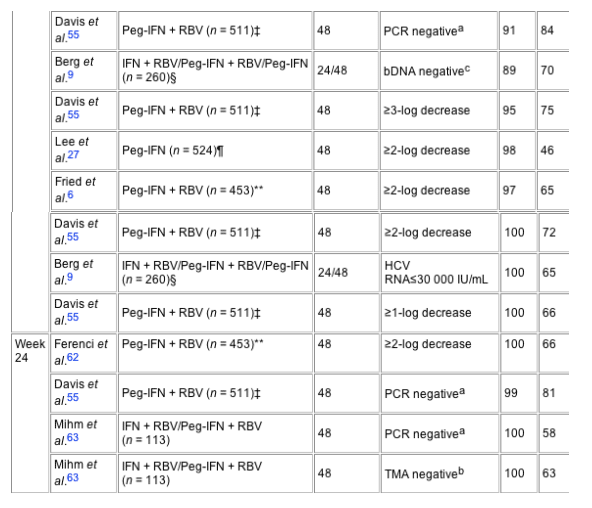
In retrospective analyses of large, randomized trials on chronic hepatitis C quantification or qualitative HCV RNA assessment after 4, 12 and 24 weeks of treatment have been used alone or in comparison with baseline HCV RNA for defining early virologic response criteria.6, 9, 27, 55, 62 All investigations provided positive and negative predictive values,6, 9, 27, 55, 62 some studies9, 27 also used odds ratio and ROC analyses to describe the early virologic response criteria. As all above-mentioned investigations focused on the development of treatment algorithms for discontinuation of therapy in patients without a chance for achieving a sustained virologic response, the negative predictive value (NPV, proportion of patients without sustained virologic response in the group of all patients without early virologic response) is of special importance. The NPV should ideally be 100% to avoid premature withdrawal of therapy in patients who might still have a chance for achieving sustained virologic response. On the other hand, patients who achieve the early virologic response criterion should also become future sustained virologic responders with a high probability as otherwise too many patients would in vain receive continued treatment [positive predictive value (PPV; proportion of patients with sustained virologic response in the group of all patients with early virologic response)]. NPV and PPVs for the early virologic response criteria of different studies6, 9, 27, 55, 62 are given in Table 2.
Table 2. Treatment algorithms for early detection and treatment discontinuation in non-responders
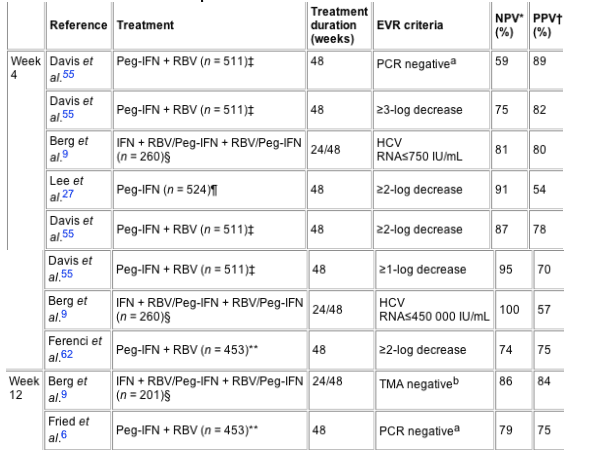
Assessment of response to antiviral therapy after 4 weeks of treatment
The more rapid HCV RNA declines during therapy the higher is the chance for the patients to achieve sustained virologic response (Table 2).9, 27, 55, 5759 If strict criteria are chosen for definition of early virologic response, such as non-detectable HCV RNA by PCR after 4 weeks of treatment, the proportion of patients with sustained virologic response in the group of all patients with early virologic response is as high as 89%.55 Although the positive predictive value using this strict criteria is 89%, the negative predictive value is only 61%.55 Therefore, the criterion of non-detectable HCV RNA by PCR at 4 weeks of treatment should not be used for early discontinuation of therapy. In a patient cohort of 260 patients treated with different interferon-alpha-based antiviral regimens, none of 140 sustained responders had HCV RNA levels above 450 000 IU/mL at treatment week 4, resulting in a NPV of 100% using this criterion. However, the PPV with this definition of early virologic response was only 57%9 and many patients without a chance for sustained virologic response would receive unnecessary treatment if this criterion was applied. In order to obtain a criterion for early virologic response leading to an optimal NPV of close to 100% and at the same time producing a reasonable PPV, HCV RNA assessment at treatment week 12 has been evaluated (Table 2).
Assessment of response to antiviral therapy after 12 weeks of treatment
The association of early virologic response at treatment week 12 with virologic response at the end of the follow-up period has been evaluated by retrospective analysis of large phase III clinical trials evaluating the efficacy of peginterferons with or without ribavirin.6, 8, 15, 32 Only two of 255 sustained virologic responders to therapy with peginterferon-alpha-2a plus ribavirin, three of 180 sustained responders to monotherapy with peginterferon-alpha-2a and none of 273 patients with sustained virologic response to treatment with peginterferon-alpha-2b plus ribavirin failed to have at least a 2-log decay after 12 weeks (Table 2).6, 27, 55 Berg et al. reported similar results: only two patients with sustained virologic response had a <2-log decay in a total cohort of 260 patients receiving different interferon-alpha-based regimens.9 As a small proportion of sustained virologic responders fails to reach a 2-log reduction in HCV RNA after 12 weeks of antiviral treatment, Berg et al. proposed an absolute value of 30 000 IU/mL at treatment week 12 to differentiate non-responders from patients with potential sustained virologic response.9 In 260 patients no sustained virologic responder had a viral load above 30 000 IU/mL (branched DNA assay) at week 12 (Table 2).9 The PPV was impaired in this analysis compared with the 2-log rule, as 54% of the non-sustained responders had <30 000 IU/mL HCV RNA. However, the cut-off value of 30 000 IU/mL has the advantage that only a single quantification is necessary and incorrect HCV RNA quantifications at baseline will not cause miscalculation of the patients' response patterns. In patients with HCV RNA levels between 30 000 IU/mL and 35 000 IU/mL at week 12, Berg et al. recommend repetition of HCV RNA quantification to further improve the negative predictive value. The rules for discontinuation of antiviral therapy after 12 weeks of treatment in patients with <2-log decay or >30 000 IU/mL are especially suitable for patients infected with HCV genotype 1 or 4. In patients infected with HCV genotype 2 or 3 these stopping rules are less suitable as >90% of these patients achieve a 2-log decline at treatment week 12 and have sustained virologic response rates of 79-84%7, 8, 33 as compared with sustained virologic response rates of 41-52% in patients with HCV genotype 1 infection.68
Assessment of response to antiviral treatment after 24 weeks of treatment
The positive predictive values of an early virologic response with an at least 2-log decline or a HCV RNA concentration of <30 000 IU/mL at treatment week 12 range from 46% to 72%.6, 9, 27, 55, 59 Thus, a substantial proportion of patients who will not achieve a sustained virologic response cannot be identified with these early virologic response criteria. Therefore qualitative assessment of HCV RNA at week 24 may be of interest, especially in HCV infected patients who show a 2-log decline or have HCV RNA levels of <30 000 IU/mL but still have detectable serum HCV RNA at treatment week 12. If qualitative PCR still remains positive for HCV RNA at treatment week 24 despite an early virologic response, treatment discontinuation is recommended as only a negligible number of sustained virologic responders will fail to achieve this criterion.9, 55, 62 Application of highly sensitive assays like qualitative transcription-mediated amplification (TMA) with a detection limit of 5-10 IU/mL at week 24 might help to identify even more patients without future sustained virologic response. In a retrospective study, 67 of 113 patients with HCV genotype 1 infection did not achieve a sustained virologic response after 48 weeks of therapy with (pegylated) interferon-alpha plus ribavirin. Of those 67 patients, 34 and 40 patients tested positive for HCV RNA at week 24 with PCR and TMA, respectively. None of the six patients who showed positive HCV RNA by TMA but not by PCR at week 24 achieved sustained virologic response after 48 weeks of therapy. All 46 patients with sustained virologic response had undetectable HCV RNA by PCR and TMA at week 24.63
Treatment algorithms for shortening of therapy in rapid responders
Although most investigations on early viral decay and treatment response focused on developing treatment algorithms for discontinuation of therapy in patients with HCV genotype 1 infection and with little or no chance for achieving a sustained virologic response, recently four clinical trials have used early HCV RNA decline data at week 4 to identify patients with rapid virologic response in whom standard therapy duration might be over-treatment;57-59, 60 (Table 3).
Table 3. Treatment algorithms for shortening of therapy in rapid responders
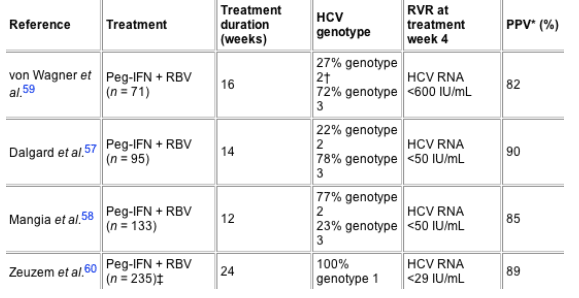
In three prospective European trials in HCV genotype 2/3 infected patients, those with negative HCV RNA testing (sensitivity 50 IU/mL,57,58 sensitivity 600 IU/mL),59 at week 4 stopped treatment at weeks 14 or 12 or 16, respectively and showed rates for sustained virologic response of 90%, 85% and 82% respectively. One of these studies59 randomized patients with rapid virologic response at week 4 into two treatment arms: one group received treatment (peginterferon-alpha-2a 180 mg/week plus RBV 1000-1200 mg/day) for 16 weeks, the other one for 24 weeks. Patients with shorter treatment duration achieved a sustained virologic response rate of 82% and patients treated for 24 weeks showed a rate for sustained virologic response of 80%. Previous studies reported rates of sustained virologic response between 79% and 84% in HCV genotype 2/3 infected patients treated for 24 to 48 weeks.7, 8, 33 However, before an individualized treatment algorithm for this patient population can be established, the data of the ACCELERATE study, including 1400 patients, should be awaited. In the ACCELERATE study all patients received 800 mg of ribavirin whereas in the three published European pilot studies weight-based ribavirin (800-1400 mg/day) was given.5759 Detailed analyses are required with respect to differences between genotypes 2 and 3, the influence of baseline viral load, the initial virologic response and the doses and durations used for peginterferon-alpha and ribavirin.
In addition to the above mentioned studies on patients with genotype 2 or 3 infection, one recent prospective clinical trial discussed shortening of treatment duration from 48 to 24 weeks in patients with HCV genotype 1 infection with a low baseline viral load of equal or <600 000 IU/mL and non-detectable HCV RNA (real-time PCR with a lower detection limit of 29 IU/mL) at treatment week 4.60 In this subgroup of patients sustained virologic response rates of 89% were achieved after treatment for 24 weeks with peginterferon-alpha-2b 1.5 mg/kg once per week subcutaneously plus ribavirin 800-1400 mg according to body weight compared with a sustained virologic response rate of 85% in the historical control group treated for 48 weeks.8
Impact of predictors for response in chronic hepatitis C on current consensus guidelines
The most recent consensus guidelines are the American Association for the Study of Liver Diseases (AASLD) practice guidelines, the Canadian consensus conference statement, and the guidelines of the German Association for Digestive and Metabolic Diseases (DGVS; all published in 2004).64 65, 66 Furthermore, the National Institute of Health (NIH) launched a consensus statement in 200267 whereas the latest EASL consensus conference dates back until 1999. However, different national European societies put forward consensus statements more recently, like the French and Scandinavian consensus guidelines in 2002.68, 69 HCV genotype and early virologic response during treatment have influenced the current consensus guidelines for antiviral therapy of chronic hepatitis C.
HCV genotype
The most important baseline predictor of virologic response is HCV genotype and all guidelines recommend that patients with HCV genotype 1 infection and those with HCV genotype 2 or 3 infection should be treated for 48 and 24 weeks respectively. Based on a large randomized multicentre trial7 in which patients received peginterferon-alpha-2a in combination with different doses of ribavirin, most consensus guidelines6467, 69 recommend to use a flat dose of ribavirin 800 mg/day in patients with HCV genotype 2 or 3 infection, whereas patients with HCV genotype 1 infection should receive ribavirin according to body weight.
Early virologic response
In patients infected with HCV genotype 1, the achievement of early virologic response is of major importance for the overall therapy success. All guidelines reviewed6469 recommend to perform a quantitative assessment of HCV RNA at baseline and week 12 of treatment. If a patient fails to show an early virologic response, i.e. at least a 2-log decrease in HCV RNA concentration from baseline, discontinuation of therapy is suggested. The AASLD guidelines, however, acknowledge that this decision may be individualized according to the severity of the underlying liver disease and the documentation of at least some virologic or biochemical response. Importantly, the Canadian consensus statement points out, that quantitative HCV RNA assays may have an inherent variability of up to 0.5 logs. Therefore, according to this guideline, the 12-week EVR rule should not be applied too strictly. The Canadian guideline suggests to accept a 1.8-log decline in HCV RNA concentration from baseline as an early virologic response. Furthermore, it seems important to use the same assays for HCV RNA quantification at baseline and week 12 as a high interassay variability might also lead to an incorrect interpretation of the patients' response patterns.70 In addition to the 12 weeks algorithm the Canadian as well as the German guidelines recommend a qualitative HCV RNA testing at week 24 in those patients who achieved an early virologic response at week 12 but did not clear HCV RNA at that time point. According to these statements, treatment should be discontinued in patients with detectable serum HCV RNA at treatment week 24. As almost all patients infected with HCV genotype 2 or 3 achieve an early virologic response, it is generally agreed that assessment of early virologic response at week 12 of therapy is not useful in this patient population.
The very recent data on shortening of treatment in HCV genotype 1 infected patients with low viral load and rapid virologic response (i.e. non-detectable HCV RNA at treatment week 4) from 48 to 24 weeks60 and to <24 weeks in patients with HCV genotype 2 or 3 infection and rapid virologic response5759 has not yet been evaluated by consensus conferences. However, based on the data of Zeuzem et al.,60 recently the European Agency for the Evaluation of Medicinal Products (EMEA) licensed combination therapy with weight-based peginterferon-alpha-2b and ribavirin for 24 weeks for the subgroup of patients with chronic HCV genotype 1 infection, a low baseline viral load (≦600 000 IU/mL) and non-detectable HCV RNA at week 4 of therapy.
Prolongation of therapy in subgroups of patients with chronic hepatitis C has not yet been addressed by consensus conferences either. Data presented at the 55th AASLD meeting in November 2004 suggest, that prolongation of therapy from 48 to 72 weeks in patients with HCV genotype 1 infection and slow virologic response (defined as HCV RNA positive at week 12 but negative at week 24) might reduce relapse rates and thereby increase sustained virologic response rates.71 Prolongation of therapy to >24 weeks might also be suitable for some patients with HCV genotype 3 infection. Within the group of patients with chronic HCV genotype 3 infection, lower sustained virologic response rates have been observed in patients with a high baseline viral load compared with patients with a low baseline viral load.33, 57, 59 Whether prolongation of therapy to >24 weeks is suitable for patients with HCV genotype 3 infection and a high baseline viral load should be investigated in prospective clinical trials.
Conclusions
Despite major improvements in anti-HCV therapy within the last years, treatment of chronic hepatitis C is still challenging and further optimization is needed. Many patient- and viral-related factors have been evaluated for an association with virologic response to interferon-alpha-based antiviral therapy. Patient-related factors have so far not lead to individualized treatment recommendations. Caution, however, should to be taken when considering shorter treatment durations in patients with advanced fibrosis or cirrhosis. Some viral factors are difficult to assess (e.g. quasispecies heterogeneity). However, viral genotype and viral load before and during therapy can be easily determined. The high predictive value for treatment success of HCV genotype as well as of the viral load before and above all during therapy allows for differentiated treatment regimen for different patient groups.
It is currently agreed, that patients with HCV genotype 2 or 3 infection are sufficiently treated with a combination of peginterferon-alpha plus ribavirin for 24 weeks regardless of the baseline viral load.7 In patients with HCV genotype 1 infection, however, quantification of the viral load is mandatory at baseline and during therapy to distinguish between patient groups with quite heterogeneous response patterns and treatment needs: patients with a low baseline viral load (≦600 000 IU/mL) and non-detectable HCV RNA (≦29 IU/mL) at treatment week 4 can be treated adequately with a combination of peginterferon-alpha plus ribavirin dosed according to body weight (800-1400 mg) for 24 weeks.60 All other patients with HCV genotype 1 infection should be treated for 48 weeks with peginterferon-alpha plus ribavirin dosed according to body weight. Patients who do not achieve early virologic response with an at least two log reduction of HCV RNA at treatment week 12 can be discontinued as they will not show sustained viral eradication even if treated for the full 48 weeks. Those patients with a 2-log reduction of HCV RNA at treatment week 12 but without viral eradication at that time point should be retested at week 24 and be discontinued as well, if HCV RNA is still detectable.
Data published this year and in 2005 suggest that further diversification of treatment regimens might be possible to optimize antiviral therapy in chronic hepatitis C. Preliminary studies show that subpopulations of patients with HCV genotype 2 and 3 infection might be sufficiently treated with <24 weeks, in particular when HCV RNA is already undetectable after 4 weeks of treatment. Furthermore, two of these studies57, 59 pointed out that patients with HCV genotype 3 infection, especially those with a high baseline viral load might have different treatment requirements, e.g. need prolonged therapy, compared with patients with HCV genotype 2 infection. These issues are addressed by current large multicentre trials. Special efforts should be put into further optimization of therapy for patients with HCV genotype 1 infection and a high baseline viral load and/or a slow response during therapy as for this patient group rates of sustained viral eradication are especially unsatisfactory. Prolongation of therapy to >48 weeks might be one way,71 combination therapies of interferon with new substances like protease inhibitors might be another promising option in these patients.
|
|
| |
| |
|
|
|