| |
SVR rates and health-related quality of life after interferon+ribavirin therapy in patients with chronic HCV and persistently normal ALT levels
|
| |
| |
Alimentary Pharmacology & Therapeutics
Volume 23 Page 777 - March 2006
E. J. BINI & S. MEHANDRU
Department of Medicine and Division of Gastroenterology, VA New York Harbor Healthcare System and NYU School of Medicine, New York, NY, USA
"....Compared with baseline, treatment was associated with significant increases in HRQOL (quality of life) in nearly all domains of the HQLQ in both groups of subjects (Table 2). As expected, the magnitude of improvement in each domain of HRQOL in the persistently normal ALT group and in the elevated ALT group was greater in patients who achieved a SVR when compared with those who did not...
.... we demonstrated that the SVR rates in HCV-infected patients with persistently normal ALT levels who were treated with interferon-a2b and ribavirin was not different from the response rates seen in subjects with elevated ALT levels.... These findings have important implications for the management of the estimated 30% of HCV-infected subjects who have persistently normal ALT levels...."
INTRODUCTION
The widespread availability of sensitive and specific antibody testing has allowed the identification of chronic hepatitis C virus (HCV) infection in asymptomatic subjects with persistently normal alanine aminotransferase (ALT) levels.1 Currently, it is estimated that 30% of patients with chronic HCV infection have persistently normal ALT levels.13 Despite the presence of normal ALT levels, HCV-infected patients can have histological evidence of hepatic fibrosis and cirrhosis.47
To date, the management of chronic HCV-infected patients with persistently normal ALT levels remains controversial.1, 8 According to the 1997 guidelines proposed by National Institutes of Health Consensus Development panel, interferon monotherapy is not recommended for this group of patients because of the low sustained virological response (SVR) rates and the risk of treatment-induced hepatic enzyme abnormalities.9 In contrast, the 2002 National Institutes of Health Consensus Development Conference on Management of Hepatitis C and the recent American Association for the Study of Liver Diseases Practice Guidelines recommend that the decision to treat HCV-infected patients with persistently normal ALT levels should be made on an individual basis.1, 8
Recommended factors that should be considered when deciding to proceed with HCV treatment in patients with persistently normal ALT levels include the severity of liver disease, HCV genotype, age, presence of comorbid disease, patient motivation and the presence of symptoms.1 In addition, the severity of impairment in health-related quality of life (HRQOL) should be considered when deciding whether to treat these patients. To date, only a few studies have evaluated the efficacy of interferon and ribavirin combination therapy in HCV-infected patients with persistently normal ALT levels. Furthermore, it is unknown whether HCV treatment improves HRQOL in this population. Therefore, we undertook the present study to determine the efficacy and safety of interferon-a2b and ribavirin combination therapy in patients with chronic HCV infection and persistently normal ALT levels, and to evaluate the effects of treatment on HRQOL.
ABSTRACT
Summary
Background
Few studies have evaluated interferon and ribavirin therapy in hepatitis C virus-infected patients with persistently normal alanine aminotransferase (ALT) levels.
Aim
To determine the efficacy and safety of combination therapy in this population, and to evaluate the impact of treatment on health-related quality of life.
Methods
Forty-six hepatitis C virus-infected patients with persistently normal ALT levels and 92 matched subjects with elevated ALT levels were treated with interferon-a2b plus ribavirin for up to 48 weeks. Health-related quality of life was measured prior to therapy and 24 weeks after completion of treatment using the Hepatitis Quality of Life Questionnaire.
Results
Overall, 32.6% of patients with normal ALT levels and 28.3% of those with elevated ALT levels had undetectable hepatitis C virus RNA at 24 weeks after completion of treatment (P = 0.60). Three patients in the normal ALT group had mild transient ALT elevations during therapy. Compared with baseline, treatment was associated with significant improvements in nearly all domains of health-related quality of life in both groups of patients.
Conclusions
In hepatitis C virus-infected patients with persistently normal ALT levels, interferon-a and ribavirin therapy is efficacious, safe, and associated with significant improvements in health-related quality of life.
RESULTS
Baseline characteristics of the patients
During the 3-year study period, a total of 46 patients with persistently normal ALT levels and 92 patients with elevated ALT levels were enrolled in this study. There were no significant differences in the demographic and clinical characteristics between the two groups at baseline (Table 1). As expected, patients with persistently normal ALT levels had a lower median necroinflammatory score than those with elevated ALT levels.
Among the patients with persistently normal ALT levels, the median ALT level was 23.0 U/L (IQR: 19.0-28.0). These individuals had a median number of normal ALT values of 8.0 (IQR: 6.0-12.0) over a median duration of time of 29.5 months (IQR: 18.8-41.0).
Efficacy of interferon and ribavirin
The full course of treatment was completed by 84.8% of the persistently normal ALT patients and 80.4% of those with elevated ALT levels (P = 0.53). Overall, the proportion of patients who had undetectable HCV-RNA at the end of treatment was similar in the persistently normal ALT patients and in those with elevated ALT levels (45.7% vs. 42.4%, P = 0.72). In addition, the end of treatment virological response did not differ when stratified by genotype (Figure 1).
A SVR was achieved in 32.6% of the patients with persistently normal ALT levels and 28.3% of those with elevated ALT levels (P = 0.60). The SVR rates between the two groups of patients did not differ when stratified by genotype (Figure 1).
Safety
Treatment with interferon-a2b and ribavirin did not cause a severe flare in ALT levels in any of the patients in the persistently normal ALT group. However, three of these subjects did develop transient elevations in ALT levels between 1.5 and 2 times the upper limit of normal during treatment; all three normalized after therapy was completed. There were no serious adverse events requiring hospitalization or deaths in either group of subjects.
Impact of treatment on HRQOL
Prior to interferon-a2b and ribavirin therapy, there were no significant differences in HRQOL in any of the HQLQ domains between the persistently normal and elevated ALT groups (data not shown). Compared with baseline, treatment was associated with significant increases in HRQOL in nearly all domains of the HQLQ in both groups of subjects (Table 2). As expected, the magnitude of improvement in each domain of HRQOL in the persistently normal ALT group and in the elevated ALT group was greater in patients who achieved a SVR when compared with those who did not (Table 3).
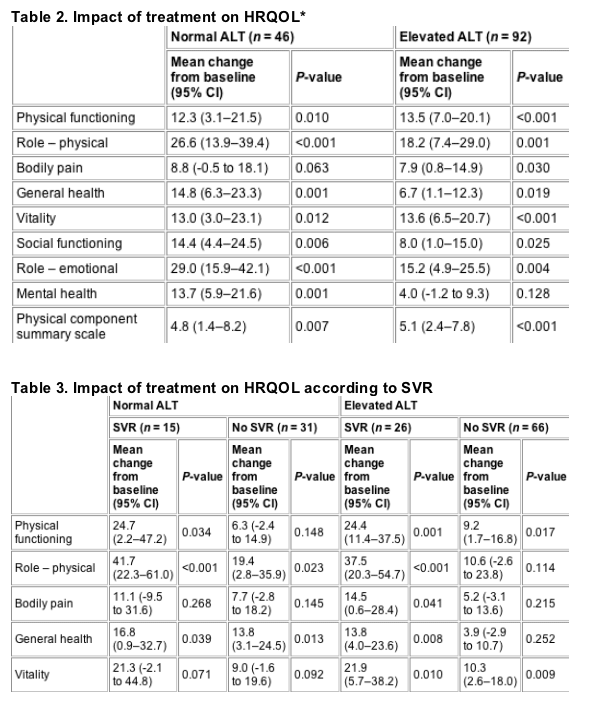
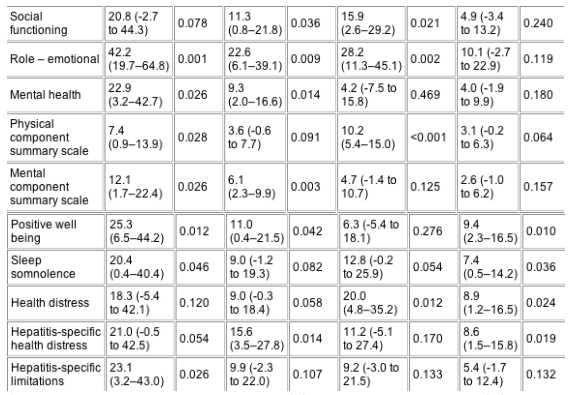
SVR, sustained virological response; HRQOL, health-related quality of life; ALT, alanine aminotransferase; CI, confidence interval.
DISCUSSION
In the present prospective controlled study, we demonstrated that the SVR rates in HCV-infected patients with persistently normal ALT levels who were treated with interferon-a2b and ribavirin was not different from the response rates seen in subjects with elevated ALT levels. Furthermore, therapy was well tolerated and was associated with significant improvements in HRQOL. These findings have important implications for the management of the estimated 30% of HCV-infected subjects who have persistently normal ALT levels.
Prior studies that have evaluated interferon-a monotherapy for the treatment of chronic HCV infection in patients with persistently normal ALT levels have enrolled a small number of subjects,1528 and only two reports included a control group of patients with elevated ALT levels.16, 17 The SVR rates with interferon-a monotherapy in subjects with normal ALT levels are approximately 10-20%,29 which is similar to what has been reported in patients with elevated ALT levels.30, 31 However, some patients with persistently normal ALT levels developed ALT flares either during or shortly after therapy with interferon, and in some subjects these changes were persistent.16, 17, 22, 2527 Based on the relatively low rates of SVR and the risk of ALT flare-ups, interferon-a monotherapy was not recommended in this population.9
The addition of ribavirin to interferon-a has resulted in SVR rates of approximately 40% in HCV-infected patients with elevated ALT levels.32 Despite the large amount of data available on interferon-a and ribavirin combination therapy for the treatment of chronic HCV infection in patients with elevated ALT levels, only a limited number of trials have been conducted in previously untreated subjects with persistently normal ALT levels.3338 Although three of these six studies included a control group of patients with elevated ALT levels,34, 36, 37 only two enrolled more than 40 subjects with persistently normal ALT levels.36, 38 Overall, the SVR rates in patients with normal ALT levels were similar to the response seen in subjects with elevated ALT levels.
In contrast to the outcomes reported in the interferon-a2b and ribavirin registration trial conducted in the US,39 our SVR rates in genotype 1 as well as in those infected with genotype 2/3 were substantially lower. This discrepancy is likely due to differences in the patient populations studied. Nearly one-third of our patients were black, and it is well known that these individuals have lower sustained response rates to therapy than non-blacks.40, 41 In addition, our subjects were older, more likely to be male, had a higher mean body weight, and had a higher proportion of subjects with cirrhosis compared with the study by McHutchison et al.39
Nonetheless, our study confirms previous findings that the SVR rates in HCV-infected patients with normal ALT levels was not different from the response rates seen in those with elevated ALT levels. Furthermore, we found that ALT flares were uncommon, mild and transient. A recent study using pegylated interferon in combination with ribavirin also confirmed the safety and efficacy of treatment in patients with persistently normal ALT levels, with 52% of subjects having a SVR after 48 weeks of therapy.42 Therefore, interferon in combination with ribavirin appears to be a safe and effective treatment option for this population of HCV-infected subjects.
The decision to treat HCV-infected patients with persistently normal ALT levels should be based on a global evaluation of the patient. As part of the treatment evaluation process, it is important to consider patient HRQOL. It is well established that HRQOL is impaired in patients with chronic HCV infection and elevated ALT levels relative to healthy controls.43 Furthermore, treatment improves HRQOL in HCV-infected patients with elevated ALT levels, especially in those who achieve a SVR.43 However, the effect of treatment on HRQOL in HCV-infected patients with normal ALT levels is unknown. In the present study, we found that treatment was associated with significant and clinically meaningful improvements in HRQOL in subjects with persistently normal ALT levels. This important finding should be considered when deciding to treat these individuals.
The strengths of the present study include the prospective study design, inclusion of a control group of patients with elevated ALT levels, and measurement of HRQOL before and after therapy using a validated instrument. However, there are some limitations of this study that should be considered when interpreting our findings.
First, the study was conducted at a single VA medical centre and the majority of our patients were men. Secondly, we did not perform follow-up biopsies to determine whether treatment improves liver histology. Thirdly, we treated patients with standard interferon-a2b plus ribavirin instead of pegylated interferon and ribavirin because pegylated interferon was not available at the time the study was designed. However, given the higher SVR rates with pegylated interferon and ribavirin when compared with standard combination therapy, it is possible that the magnitude of improvement in HRQOL with pegylated interferon and ribavirin treatment would be even greater. Finally, patients were not blinded to their viral response and this could have influenced the HRQOL outcome data.
In conclusion, our study demonstrates that treatment of HCV infection with interferon-a2b and ribavirin combination therapy is efficacious, safe, and results in significant improvements in HRQOL in patients with persistently normal ALT levels. The present study adds to the growing body of evidence that suggests that patients with chronic HCV infection should not be excluded from treatment on the basis of normal ALT levels alone. In addition to the presence of fibrosis and other clinical criteria, reduced HRQOL should be considered as an indication for treatment in this population.
Methods
Study population
Eligible patients were enrolled in this study at the Veterans Affairs (VA) New York Harbor Healthcare System in New York City between January 1999 and December 2001. Adult patients who had not previously been treated with interferon or ribavirin were eligible for the study if they had a positive HCV antibody test, had detectable serum HCV-RNA by polymerase chain reaction (PCR; COBAS Amplicor HCV Monitor Test version 2.0, Roche Molecular Systems, Branchburg, NJ, USA), had findings consistent with a diagnosis of chronic HCV infection on liver biopsy performed within 1 year of enrolment, and had persistently normal ALT levels. Persistently normal ALT levels were defined as at least three normal ALT levels (<40 U/L) on separate occasions at least 1 month apart over a 6-month period. Subjects were not considered as having persistently normal ALT levels if they ever had any documented ALT level above the upper limit of normal.
For each patient with persistently normal ALT levels, we enrolled two HCV-infected patients with elevated ALT levels matched for genotype, HCV viral load (< or ≥2 million copies/mL), and the presence of cirrhosis. After enrolment of each normal ALT patient, we enrolled the next two elevated ALT patients who met our eligibility criteria and who were to begin therapy. The same inclusion criteria listed above were used for enrolment of the patients with elevated ALT levels.
Patients were excluded from this study if they had coinfection with the hepatitis B virus or the human immunodeficiency virus, neutropenia (neutrophil count: <1500/mm3), anaemia (haemoglobin: <13.0 g/dL in men or <12.0 g/dL in women), thrombocytopenia (platelets: <75 000/mm3), a serum creatinine level more than 1.5 times the upper limit of normal, an a-fetoprotein concentration of more than 50 ng/mL, decompensated cirrhosis, prior organ transplantation, neoplastic disease, severe cardiac, pulmonary, or other comorbid diseases, poorly controlled diabetes or thyroid disease, autoimmune diseases, seizure disorders, history of concurrent immunosuppressive therapy, or a history of consumption of more than 10 g of alcohol per day or use of illicit drugs within 6 months of enrolment. This study was approved by our local Institutional Review Board, and all patients provided written informed consent.
Study design
Percutaneous liver biopsies were performed using a Menghini needle. Biopsy specimens were fixed in formalin, embedded in paraffin, and stained with haematoxylin-eosin and Masson trichrome stains. Biopsy specimens were evaluated by a board-certified staff pathologist who was blinded to the aims of this study. The necroinflammatory score of liver disease was graded on a scale of 0-18 using the Ishak modification of the Histologic Activity Index,10 whereas fibrosis was scored on a scale from 0 to 4 using the Scheuer staging system.11
Patients were treated with Food and Drug Administration approved doses of Rebetron (Schering-Plough, Kenilworth, NJ, USA), which included interferon-a2b 3 million units subcutaneously three times per week and ribavirin 1000 mg (weight <75 kg) or 1200 mg (weight ≥75 kg) by mouth per day. Patients with HCV genotypes 2 or 3 were treated for 24 weeks, while those with HCV genotype 1 were treated for up to 48 weeks. In patients with HCV genotype 1, therapy was stopped after 24 weeks if HCV-RNA was still detectable by PCR (COBAS Amplicor HCV Test version 2.0, Roche Molecular Systems; sensitivity 100 copies/mL). HCV genotyping was performed using the Inno-LiPA HCV II assay (Innogenetics, Gent, Belgium).
Patients were followed at weeks 2, 4, and then every 4 weeks during treatment, and at 4, 8, 12 and 24 weeks after cessation of therapy. At each visit, the presence and severity of adverse events were assessed by history and physical examination and a comprehensive panel of laboratory tests (complete blood count, serum electrolytes and liver profile).
Health-related quality of life was assessed prior to treatment initiation and at 24 weeks after completion of therapy using the validated Hepatitis Quality of Life Questionnaire (HQLQ).12, 13 The HQLQ includes eight generic domains from the Medical Outcomes Study Short Form-36 survey, along with three additional generic domains thought to be relevant to those with chronic HCV infection (positive well being, sleep somnolence and health distress) and two hepatitis-specific domains (hepatitis-specific health distress and hepatitis-specific limitations). Each domain is scored from 0 to 100, with higher scores indicating better HRQOL. In addition, the physical component summary and mental component summary scales were scored using standard algorithms.14 In contrast to the 0-100 scores used for the other domains, the physical component summary and mental component summary scales were standardized using norm-based scoring to have a mean of 50 and a standard deviation of 10 in the general United States population. Therefore, any score <50 is considered below the general population mean, and each point represents 1/10th of a standard deviation.
Study outcomes
The primary end point of this study was a SVR to treatment, defined as undetectable HCV-RNA (<100 copies/mL) using the COBAS Amplicor HCV Test version 2.0 at 24 weeks after completion of therapy. Secondary end points of this study included a virological response (undetectable HCV-RNA) at the end of treatment, the safety of HCV therapy in these populations and the change in HRQOL from baseline. All virological end points were evaluated by intention-to-treat analysis, and the safety analysis included all patients who received at least one dose of study medication.
Statistical analysis
Continuous variables were compared using the unpaired two-tailed t-test or the Mann-Whitney U-test, as appropriate. Changes in HRQOL from baseline were analysed using a paired samples t-test. For all continuous variables, data are expressed as mean ± s.d. for those variables that were normally distributed, and medians with interquartile ranges (IQR; 25-75th percentiles) for those with a non-normal distribution. Categorical variables were compared using the chi-squared test or Fisher's exact test. Statistical analysis was performed using spss software version 13.0 for Windows (SPSS Inc., Chicago, IL, USA) and a two-tailed P-value of <0.05 was considered statistically significant.
|
|
| |
| |
|
|
|