| |
HCV Therapy Successful for With Mental illness/substance abuse
|
| |
| |
"Comparison of hepatitis C treatment patterns in patients with and without psychiatric and/or substance use disorders"
Journal of Viral Hepatitis
Volume 13 Page 235 - April 2006
S. Chainuvati1,2,*, S. K. Khalid1,2,*, S. Kancir3, M. Shea1,2, J. Edwards2, M. Sernyak2,4, S. Wongcharatrawee1,2 and G. Garcia-Tsao1,2
With the objective of evaluating the validity of the assumption that patients with psychiatric illness and/or SU have a poorer completion and response to therapy, we compared treatment eligibility, treatment completion and response to antiviral therapy in veterans with chronic hepatitis C with mental illness (MI) and/or SU and those without these comorbidities.....HCV-infected veterans with MI(mental illness)/SA(substance abuse) are being offered therapy and treated less often than those without such co-morbidities, however therapy completion and SVR rates are similar, challenging the perception that adherence is poorer in this patient population.... Therefore, this study supports the conclusions of the most recent NIH Consensus conference in that 'efforts should be made to increase the availability of best current treatments to patients with neuropsychiatric co-morbidities' and that 'a history of alcohol abuse or active injection drug use should not be used to exclude patients from antiviral therapy'....In conclusion, our retrospective study evaluating patterns of therapy in HCV-infected veterans with psychiatric and/or SU disorders should encourage the referral of these patients to clinics that involve close collaboration between physicians experienced in treating HCV infection and specialists in the assessment and treatment of SU and depression. Prospective studies are ongoing to determine predictors of completion of therapy and response among these special patient populations."
Summary. Hepatitis C virus (HCV) infection is more frequent in veterans than in nonveterans. Up to 85% of HCV-infected veterans have psychiatric and/or substance use (SU) co-morbidities which, prior to the 2002 NIH Consensus Conference, were considered relative contraindications to antiviral therapy, assuming a poor adherence. With the objective of evaluating the validity of this assumption, we compared eligibility, completion and response to antiviral therapy in HCV-infected veterans with and without these comorbidities. Veterans who were anti-HCV-positive and had been seen at least once in the liver clinic (between October 1999 and June 2002) were identified through the CT-VAHCS database. Records were reviewed for patient demographics and status of liver disease, assessment of treatment eligibility, type of therapy, completion of therapy and virological response. Patients with active mental illness (MI) or SU were compared with those without these comorbidities (controls). Of 697 anti-HCV-positive-patients, 647 HCV-RNA-positive patients were included, 294 with MI/SA and 353 controls. Patient demographics, viral and liver disease characteristics were comparable between groups. Patients with MI/SA were considered ineligible for therapy more frequently (53%vs 39%, P < 0.001) and were treated less frequently (21%vs 28%, P = 0.03) than controls. However, completion of therapy (72%vs 59%) and sustained virological response (SVR) (20%vs 25%) did not differ significantly between groups. HCV-infected veterans with MI/SA are being offered therapy and treated less often than those without such co-morbidities, however therapy completion and SVR rates are similar, challenging the perception that adherence is poorer in this patient population.
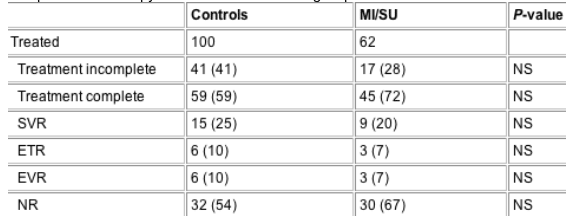
Table 3 Treatment completion and response to antiviral therapy
completion of therapy did not differ between groups.
MI, mental illness; SU, substance use; SVR, sustained virological response; ETR, end-of-treatment response; EVR, early virological response; NR, nonresponse.
Table 4 Reasons for incomplete antiviral therapy
Depression was the psychiatric diagnosis in all of the patients with MI who did not complete therapy
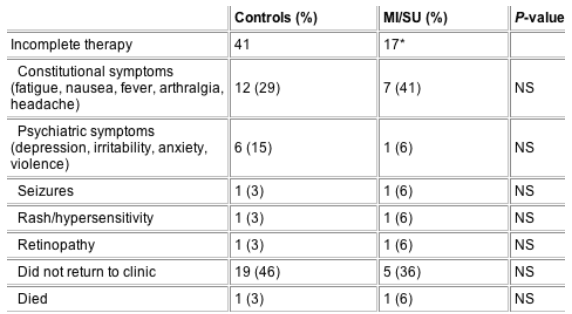
*14 patients had mental illness (MI) alone (all depression), two had substance use (SU) (opiates) + MI (depression) and the remaining patient had SU alone (opiates).
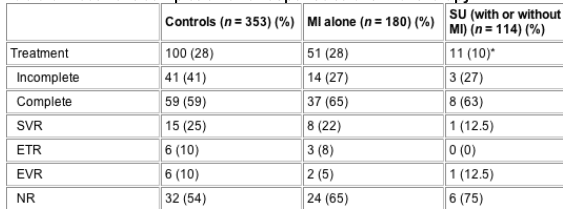
Table 5 Treatment completion and response to antiviral therapy
MI, mental illness; SU, substance use; SVR, sustained virological response; ETR, ; EVR, early virological response; NR, nonresponder.
*P = 0.0001 when compared with control group. Otherwise, comparisons among groups were not significantly different.
Introduction
Hepatitis C virus (HCV) infection is a major health problem in the United States with an overall prevalence of antibodies to HCV of 1.8%, corresponding to approximately 3.9 million individuals, and a 1.3% prevalence of positive HCV-RNA, that is, an estimated 2.7 million with a chronic infection [1,2]. In United States veterans, the prevalence of anti-HCV positivity is much higher, with reported rates ranging between 7% and 35% [3-6] with a more recent study demonstrating a 5.4% prevalence [7] and rates of chronic infection of 8-16% [5]. Treatment of hepatitis C has evolved favourably over the years and recent controlled trials using the combination of pegylated interferon plus ribavirin have shown viral eradication rates ranging between 54% and 61% [8-10]. In the most recent National Institutes of Health Consensus Conference [11], this combination was considered the most effective and therefore, the recommended therapy for hepatitis C. It also established that patient adherence is critical to the success of HCV therapy.
Until recently, psychiatric and substance use (SU) comorbidities had been considered contraindications to antiviral therapy for hepatitis C [12-14], because it has been assumed that these patients would have decreased adherence to therapy and a higher rate of neuropsychiatric side-effects both of which would hinder completion of therapy and thereby the possibility of viral eradication. Unfortunately, psychiatric, drug- and alcohol-use disorders are present in up to 85% of HCV-infected veterans [15] and therefore a majority of veterans have been excluded from antiviral therapy. Also, a high prevalence of HCV infection (8.5%) has been found in a psychiatric population [16].
With the objective of evaluating the validity of the assumption that patients with psychiatric illness and/or SU have a poorer completion and response to therapy, we compared treatment eligibility, treatment completion and response to antiviral therapy in veterans with chronic hepatitis C with mental illness (MI) and/or SU and those without these comorbidities.
Results
The data set examined 1750 unique patients with a positive anti-HCV at the CT-VAHCS between October 1999 and June 2002. Of these, 697 patients were identified as having at least one visit to liver clinic; 50 of whom were excluded from further analysis because of a negative HCV-RNA. Therefore, 647 patients comprise the study, 353 controls and 294 patients with MI/SU: 180 with MI, 62 with SU and 52 with both MI and SU. Of 232 patients with MI, 47% had PTSD, 13% depression, 8% bipolar disorder, 5% had schizophrenia and 27% had a combination of two or more diagnoses, mostly PTSD + depression. Of 114 patients with SU, 77% used alcohol, 5% IVD and 18% used both alcohol and IVD.
Median age of the 647 patients was 51 (range 32-82), the majority (624 or 96%) were male, 70% were Caucasian and 22% were African American. As shown in Table 1, there were no differences in baseline characteristics between study groups, although there was a tendency for a lower proportion of patients with MI/SU undergoing liver biopsy. Eighteen patients (3%) had received prior treatment for hepatitis C; 14 with interferon monotherapy and four with standard interferon with ribavirin.
Overall, of 647 patients, 296 (46%) were considered ineligible for therapy by the treating hepatologist, while the rest (54%) were considered eligible for therapy. However, only 162 (25% of total) received antiviral therapy. The most common reasons to consider patients ineligible for therapy were active MI and/or SU (n = 68, 23%), decompensated cirrhosis (n = 63, 21%), persistently normal ALT (n = 60, 20%), and significant non-hepatic disease (n = 32, 11%). As shown in Table 2, patients in the MI/SU were considered ineligible for therapy significantly more frequently than controls and, consequently, a lower proportion of patients with MI/SU received antiviral therapy. The type of psychiatric disorder did not influence treatment eligibility. While decompensated cirrhosis was a more common reason for treatment ineligibility in the control group, active MI or SU were, as expected, frequent reasons to exclude patients in the MI/SU group from therapy. Of 189 patients who did not receive therapy despite having been considered eligible by the treating physician and having been offered therapy, the most common reasons were failure of the patient to return to the clinic (n = 74, 39%), patient refusal or deferral (n = 61, 32%) and patients in whom pretreatment workup (e.g. liver biopsy, HCV quantitation or genotype testing, etc.) (n = 40, 21%) had not been completed. Of note, and as shown in Table 2, a significantly greater proportion of patients in the MI/SU group had not yet started therapy because workup was ongoing at the time of termination of the study.
Of 351 patients considered eligible for therapy, 162 patients received antiviral therapy (100 patients in the control group, 62 patients with MI/SU): 30 (18%) received interferon monotherapy, 116 (72%) received interferon plus ribavirin and 16 (10%) were started on pegylated interferon plus ribavirin. No differences in type of therapy were observed between study groups.
Of 162 treated patients, 104 (64%) completed therapy and as shown in Table 3, completion of therapy did not differ between groups. Only 40 (38%) patients who completed therapy had HCV genotyping (36 genotype 1, 4 genotype non-1). A SVR was observed in 24 (23%) of the patients who completed therapy and rates did not differ either between study groups (Table 3). Of the 24 patients with a SVR, HCV genotype was 1 in two patients, genotype 2 in another two and in the remaining 20 patients, genotyping was not performed.
Reasons for incomplete therapy in 58 patients (41 controls, 17 MI/SU patients) are shown in Table 4. No differences were found between study groups. Importantly, psychiatric side-effects were not greater (and actually tended to be lower) in the MI/SU group. Depression was the psychiatric diagnosis in all of the patients with MI who did not complete therapy (Table 4). Two deaths occurred during therapy, one (in the control group) was a sudden cardiac death that was considered unrelated to therapy and the other (in the MI/SU group) was a death from an unknown cause.
When the MI/SU group was divided into patients with MI alone (n = 180) and those with SU (with or without MI) (n = 114) (Table 5) it is apparent that it was mainly the SU patients that were treated significantly less often than controls, however the rate of completion of therapy and SVR were not significantly different among the three groups. Of the 11 treated SU patients, nine were alcohol users (one of whom had a SVR) and two were IVD users (none with an SVR). No patient with both alcohol and IVD use was considered eligible for therapy.
Discussion
The main findings of our study is that veterans chronically infected with HCV who have psychiatric and/or SU disorders have been treated less often than those without these comorbidities, however when these patients receive antiviral therapy, their rates of treatment completion and viral eradication are comparable with those of veterans without these disorders. Therefore, this study supports the conclusions of the most recent NIH Consensus conference in that 'efforts should be made to increase the availability of best current treatments to patients with neuropsychiatric co-morbidities' and that 'a history of alcohol abuse or active injection drug use should not be used to exclude patients from antiviral therapy' [11].
Although the veteran population is not representative of the general population mainly because of its male predominance, there is no data to indicate that gender is a determinant of adherence or response to antiviral therapy. Despite the obvious limitations of a retrospective study, our series has the advantage of including a larger number of HCV-infected veterans than other similar series [22-25] and management in a liver clinic with homogeneous criteria. We confirm that only a minority of veterans receive antiviral therapy. Of 697 patients seen in liver clinic, half were considered eligible and were offered antiviral therapy, however only half of them (25% of the whole population) underwent therapy. This low percentage of treated patients is comparable to recent studies in veterans. The study by Cawthorne showed that, of 242 patients seen in the liver clinic, only 77 (32%) were suitable candidates and were started on therapy. The study by Muir and Provenzale describes a treatment eligibility rate of 32% in 100 evaluated patients [24], although it is unclear in this study whether all eligible patients went on to receive therapy. As in other series [23,24], MI and/or SU were frequent reasons for ineligibility. Interestingly, while almost half of the patients with active SU were considered ineligible for therapy, this occurred in only a minority of patients with active MI. Another main reason to consider patients ineligible for therapy was the presence of decompensated cirrhosis, indicating that veterans with HCV infection present to the liver clinic with advanced stages of disease. In fact, in 38% of patients in whom a liver biopsy was performed, the histological stage was 3-4, similar to what has been previously reported [25].
Adherence to antiviral therapy is of major importance in achieving a SVR [26]. Hepatitis C patients with MI and SU have been routinely excluded from antiviral therapy, with one assumption being that these patients would not effectively adhere to and complete antiviral treatment. Our study shows that rates of completion of therapy were similar in both groups, and in fact there was a tendency for a higher completion rate (72%) in MI/SU patients than in controls (59%). This can perhaps be accounted for by the fact that MI/SU patients were being simultaneously seen in psychiatric clinics and were receiving psychological support during therapy.
Also, patients with MI/SU included in the study had been referred to the liver clinic and had complied with the referral, thereby selecting a group of patients with 'stable' psychiatric disorders and a theoretical greater adherence. It is important to note however, that all these patients had an axis I DSM-IV diagnosis and therefore patients with mild depression are not included. Although patients in the control group did not undergo formal psychiatric evaluation and active MI/SU may have been underestimated in this group, it is unlikely that an active major psychiatric illness would have been missed during the initial liver clinic evaluation.
An interesting finding in this study was that losses to follow-up before or during therapy were significantly greater in controls (69/353, 19%) than in patients in the MI/SU group (29/294, 10%) (P = 0.001). This is in accordance with a prospective study that showed that psychiatric patients had the best compliance and a low dropout rate [27] and can again be explained on the basis that MI/SU patients were simultaneously being evaluated in the psychiatric/mental health clinics, and were receiving the psychological support required during evaluation and treatment. MI and SU patients are thus expected to maintain compliance when there is a simultaneous collaboration with a psychiatric provider as has been demonstrated in other studies [19,20,22,27].
Treatment with interferon-based therapies is associated with numerous neuropsychiatric side-effects that may be enhanced in patients with pre-existing psychiatric conditions. Although data on side-effects during therapy was not collected, side-effects that would lead to discontinuation of therapy (i.e. sever adverse events) were not different between study groups; specifically there were no difference in severe neuropsychiatric side-effects leading to therapy discontinuation. This is in accordance with the study by Ho et al. [22], performed in 33 veterans with hepatitis C (14 with a psychiatric diagnoses and 19 without such a diagnosis) that showed that although neuropsychiatric symptoms had a tendency to be higher in psychiatric patients (32%vs 14%, NS), major neuropsychiatric side-effects requiring discontinuation of treatment was similar in both groups (14%vs 16%) and similar to the rates observed in our study. Furthermore, a recent prospective study that included 58 patients with psychiatric disorders, IVD users on methadone and former drug addicts, showed no differences in the development of depression during therapy with interferon and ribavirin compared with controls [27]. Nevertheless, depression was the diagnosis in all our patients with MI who did not complete therapy, and although they did not implicate worsening depression as cause for treatment discontinuation, this may be the underlying cause and this issue needs further exploration in prospective studies.
We also confirm that HCV-infected veterans have a SVR rate substantially lower than previously reported in randomized clinical trials. Although data on SVR may be limited by the retrospective nature of this study, known baseline predictors of SVR such as fibrosis stage, HCV genotype and prior antiviral treatment were equally distributed between groups as was the specific type of antiviral treatment administered. In our study, the majority of patients were treated with standard interferon and ribavirin, with which SVR rates achieved in controlled trials is 38-43% [28,29]. These trials have all been analysed on an intention-to-treat basis and rates of completion of therapy are in the order of 80% [26]. In our study, completion of therapy was lower (64%) and SVR was achieved in 23% of patients who completed therapy and in only 15% (24/162) of all treated patients (intention-to-treat). Even lower SVR rates of around 15% (of patients who completed therapy) have been demonstrated in other studies in which the patient population includes a large proportion of African Americans [23,30], a patient population that has been shown to have a lower response rate [31]. SVR in Caucasian veterans who complete therapy with combination interferon/ribavirin have been described as being between 26% and 28% [25,30], similar to our findings. Low SVR rates in veterans are probably due to the presence of poor predictors of response in a substantial percentage of patients, such as predominance of genotype 1 and advanced stages of fibrosis. An additional factor could be sub-optimal doses of interferon and/or ribavirin, either through dose reductions or poor adherence (i.e. missing doses), although patients were closely and regularly observed by physicians and a nurse practitioner in a dedicated liver clinic and were consistently encouraged to adhere to therapy.
Although SVR rates were low, they were not different between patients with MI/SU and controls, dispelling the concern that patients with MI and/or SU is are less likely to clear virus. However, among patients with MI/SU, SVR had a tendency to be lower in those with SU, although only 11 patients with SU received therapy and this issue should be further explored.
In conclusion, our retrospective study evaluating patterns of therapy in HCV-infected veterans with psychiatric and/or SU disorders should encourage the referral of these patients to clinics that involve close collaboration between physicians experienced in treating HCV infection and specialists in the assessment and treatment of SU and depression. Prospective studies are ongoing to determine predictors of completion of therapy and response among these special patient populations.
Materials and methods
Patients
The study population was identified through the computerized database of the CT-VA Healthcare System (CT-VAHCS). A cohort of unique patients seen at the West Haven or Newington campuses, who fulfilled the following criteria were included in the study: (i) anti-HCV positive; (ii) seen at least once in a general liver clinic between October 1999 and June 2002. The cohort was divided in two groups for analysis: (a) patients with active MI or SU and (b) patients without these comorbidities. Follow-up ended in June 2003 when a prospective study aimed at treating patients with active MI and SU was initiated. Median follow-up of patients was 43 months.
Patients with active MI were those who attended one or more of the MI clinics, had a documented active axis I DSM-IV psychiatric diagnosis (e.g. depression, schizophrenia, post-traumatic stress disorder, etc.) [17] and were receiving treatment for a psychiatric disorder at the time of evaluation in the liver clinic. Patients with SU were those who had a recognized illicit drug use problem (attending methadone clinic) and/or a recognized alcohol use problem (attending either a alcohol rehabilitation clinic or having a positive CAGE) within 1 year of evaluation in the liver clinic. Patients were assessed for antiviral therapy in the liver clinic by a gastroenterologist/hepatologist. Eligibility for antiviral therapy was mostly determined based on the recommendations of the 1996 NIH Consensus conference [12] and the Veterans Administration treatment guidelines (version 1) [18]. However, based on evidence in the literature [19,20] suggesting that the recommendation of excluding patients with active MI was unwarranted, patients whose MI/SU was considered 'stable' were considered for therapy. Patients were considered ineligible for therapy if they had unstable MI, active SU (illicit drug use or any alcohol use within 1 month prior to evaluation), decompensated cirrhosis, persistently normal alanine aminotransferase (ALT) levels, significant nonhepatic diseases, cytopenias, autoimmunity, hepatocellular carcinoma, HIV and age >65 years. Antiviral therapy (type and duration) recommended was the best available at the time of evaluation.
Data collection
Data were collected through chart review of the study cohort. Besides demographic data (age at time of first visit to the liver clinic, gender, ethnicity), charts were reviewed for the presence of MI, SU and medical comorbidities. The following laboratory tests were recorded for each patient: anti-HCV by ELISA, HCV-RNA and HCV genotype, liver biopsy and stage of fibrosis (using METAVIR score [21]). Assessment of treatment eligibility (or ineligibility) was obtained from clinic notes. For patients who did not receive antiviral therapy, stated reasons for not being treated were recorded. For patients who received treatment, the specific treatment, its duration and the outcome of therapy were recorded. For patients failing to complete therapy, stated reasons for premature discontinuation (i.e. incomplete therapy) were recorded.
Therapy was defined as complete in patients who received therapy for a period ≥80% of the planned duration of therapy; patients who received therapy for <80% of the planned duration were considered as having an incomplete therapy. A sustained virological response (SVR) was defined as a nondetectable HCV RNA 24 weeks after discontinuation of therapy. An end-of-treatment (ETR) response with pending SVR was defined as a nondetectable HCV RNA at the end of treatment but 24 weeks not having elapsed to assess SVR. An early virological response (EVR) was defined as an undetectable HCV-RNA at 12 weeks of therapy in patients in whom therapy was ongoing at the time follow-up was terminated. A nonresponse (NR) was defined as a detectable HCV RNA after completion of a minimum of 24 weeks of therapy.
Statistical analysis
Univariate analyses were used to compare data between the two study groups. Nonparametric statistics were used, Fisher's exact test was used for dichotomous variables, while Kruskal-Wallis test was used to compare continuous variables. Data collection was performed prior to subgroup allocation. Results are expressed as medians and ranges.
|
|
| |
| |
|
|
|