| |
Hepatitis C Virus Infection in African Americans
|
| |
| |
Download the PDF here
Clinical Infectious Diseases Jan 1, 2006;42:82-91
Brian L. Pearlman
Center for Hepatitis C, Atlanta Medical Center, Medical College of Georgia, and Emory University School of Medicine, Atlanta, Georgia
"....HCV infection is more prevalent in the African American population than in any other racial group in the United States (table 1). Although African Americans represent only 12% of the US population, they represent 22% of the estimated Americans with chronic HCV infection....
....African American persons have lower rates of sustained virologic response to pegylated IFN combination therapy than do white persons, even when controlling for genotype 1 infection..." (studies report- 2% vs 12% for standard interferon, 16% vs 32% for interferon+ribavirin, 19-26% for peginterferon+ribavirin) see discussion and study results below)
"...A multitude of hypotheses have been promulgated to explain the dissimilar treatment responses among ethnic groups....dysregulated CD4 function has been described in African Americans. Other possibilities include discrepant viral kinetics, cytokine production, and iron stores...." See discussion below
".....Later in 2006, final results are expected from a multicenter study sponsored by the National Institute of Diabetes and Digestive and Kidney Disease. This trial, called the Viral Resistance to Antiviral Therapy for Chronic Hepatitis C (VIRAHEP-C), has enrolled about 200 African American subjects and about 200 white subjects who will be treated with pegylated IFN and ribavirin. The purposes of the study are to assess response rates to therapy, as well as to analyze both viral factors and host factors, including genetic and immunologic variables that may influence treatment results...."
"....One hundred eighty HCV/HIV-coinfected patients in an inner city area were evaluated for suitability for combination therapy with IFN plus ribavirin. African Americans were more than twice as likely to be ineligible than eligible for HCV treatment. Concomitant medical problems, psychiatric problems, poor adherence to treatment, and/or ongoing substance abuse were factors that led to patients being ineligible for therapy...."
ABSTRACT
Hepatitis C is more prevalent among African Americans than among persons of any other racial group in the United States. However, comparatively little data are available on the natural history and treatment of hepatitis C in this population. Compared with white persons, African American persons have a lower rate of viral clearance and, consequently, a higher rate of chronic hepatitis C. Nonetheless, African American persons may have a lower rate of fibrosis progression than do white persons. African American persons with hepatitis Crelated cirrhosis have higher rates of both hepatocellular carcinoma and liver cancerrelated mortality than do white persons with hepatitis Crelated cirrhosis. In nearly all treatment trials that enrolled a significant proportion of African American subjects, such patients had inferior treatment responses, compared with those of white subjects. The prevalence of infection with hepatitis C virus genotype 1 is higher among African American patients than white patients, although this difference does not account for a greatly dissimilar response to therapy. Some of the postulated mechanisms for these disparate treatment responses and natural histories of infection are also reviewed.
Introduction
Hepatitis C virus (HCV) infection is a major public health problem for persons of all races, and it has become the most common cause of death associated with liver disease in the United States [1]. According to population-based studies, HCV infection accounts for >10,000 deaths per year [2]. Furthermore, the number of HCV-related deaths is expected to triple by the year 2020 [3].
African Americans experience complications of some chronic diseases disproportionately, compared with their white American counterparts [4, 5], and they are often underrepresented in clinical trials [6]. These differences also exist with respect to HCV infection; African American subjects represent only 5%-10% of participants in clinical trials involving HCV infection. Moreover, clinical features, such as the natural history of infection, infection prevalence, and therapeutic response, are disparate among minority and majority populations.
The African American population in the United States has a dominant ancestry from sub-Saharan West Africa [7]. However, the term "African American" has been criticized because of its imprecise geographic and cultural meaning. Furthermore, a racial classification does not necessarily convey genetic homogeneity [8]. Despite these limitations, the term "African American" will be used throughout this survey.
The aim of this review is to highlight the discrepancies in HCV infection characteristics and treatment responses between African American and white persons in the United States.
EPIDEMIOLOGY, GENOTYPE, AND NATURAL HISTORY
According to the most recent US census data, 12% of the population is African American, whereas 75% is white [9]. HCV infection is more prevalent in the African American population than in any other racial group in the United States (table 1). Although African Americans represent only 12% of the US population, they represent 22% of the estimated Americans with chronic HCV infection [3].
Table 1. Rates of hepatitis C seroprevalence among African American and white populations.
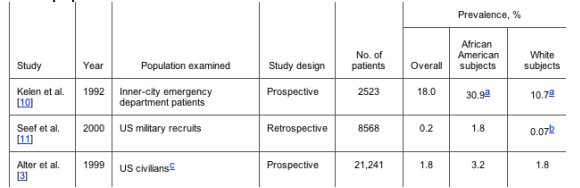
a Sample of men; no women were included in the sample.
b Likely includes Hispanic subjects.
c Subjects were noninstitutionalized persons.
The mode of transmission of HCV appears to be similar for white and African American individuals. In a retrospective chart review of 355 patients with chronic HCV infection, injection drug use was the most common means of transmission for both ethnic groups, followed by receipt of a contaminated blood transfusion. In 25% of patients, irrespective of race, the mode of transmission was unknown [12]. In a prospective, controlled treatment trial involving >400 patients, injection drug use was also the predominant means of transmission (48%50% of cases) in subjects of both races [13].
The prevalences of HCV genotypes also differ among racial groups. Although 70% of overall HCV isolates in the United States are of genotype 1 [14], there is a higher prevalence of genotype 1 infection among African Americans than among any other racial group (table 2). The explanation for this disparity is currently unknown.
According to the Center for Disease Control and Prevention's sentinel surveillance data on viral hepatitis [16], there has been a significant decrease in the number of cases of acute HCV infection since 1989. This decrement was seen in all ethnic and racial groups studied [17]. Between 1991 and 1996, African American patients accounted for 10% of patients with acute HCV infection. With respect to acute infection clinical features, African American and white subjects had nearly identical elevations in aminotransferase levels and in rates of jaundice and death [18].
Although the incidence of acute HCV infection does not seem to vary between races, the chronic HCV infection rate is higher among African American than among white individuals (table 3). Despite higher rates of chronic infection, HCV-infected African American persons may have a slower rate of fibrosis progression, compared with their white counterparts. In a retrospective chart review of 355 patients who underwent liver biopsy at a university medical center, the authors found significant differences between African American and white patients that could not be explained by age, alcohol use, or duration of infection [12]. The study suggests that histologic progression of HCV infection occurs less rapidly among African American patients than among white patients; however, there are obvious limitations of a retrospective analysis of disease progression. Furthermore, 19% of the nonAfrican American patients were Hispanic, and a subgroup analysis was not separately performed. This factor is especially important, because Latino persons may have a faster rate of liver fibrosis than do either African American or non-Hispanic white persons [21, 22]. Nonetheless, other studies support the notion that African American persons may experience slower histologic progression than do white persons (table 4).
Prospective, randomized controlled trials are needed to better clarify the natural history of infection in African American persons. Discrepancies in disease severity may not necessarily correlate with differences in disease progression, as has been seen in cross-sectional and retrospective studies. Furthermore, not all studies have confirmed that natural histories of infection are dissimilar between races; preliminary data from a large multicenter treatment trial of patients with chronic HCV infection show no difference between African American and white patients with regard to the rate of fibrosis [25].
The mechanism for the possible discrepancies in the natural history of hepatitis C is unknown; however, the answer may lie in disparate HCV-specific CD4 T cell responses between African American and white persons. The strength and sustenance of HCV-specific T cell responses have already been identified as critical determinants of viral clearance during acute HCV infection [2628]. In a clinical and immunologic analysis of 99 HCV-infected patients, CD4-proliferative T cell responses were observed in response to HCV-derived antigens in African American and white participants with both viral persistence and spontaneous clearance. Compared with chronically infected patients with a relatively weak HCV-specific T cell response, patients who achieved HCV clearance had a robust T cell response, irrespective of race. However, compared with chronically infected white patients, chronically infected African American patients had a significantly greater T cell proliferative response to HCV [29]. Futhermore, acute HCV infection clearance requires a potent IFN- response [30]. In African American patients, HCV-specific CD4 T cell proliferative responses were not accompanied by IFN- production, suggesting a dysregulated, virus-specific T cell function in cases of chronic infection in this population. The authors concluded that there are novel ethnicity-related differences in CD4 T cell responsiveness to HCV [29].
Other mechanisms invoked to explain the disparities in the natural history of hepatitis C among different races include the stronger association of certain human leukocyte antigen class II alleles with HCV clearance in African Americans [30] and the lack of immune system recognition of the virus in African Americans [12]. In a recent study, variants of the immunomodulatory IL-10 and IL-19/20 genes seemed to play a role in the spontaneous clearance of HCV in African American patients; no such relationship was found in white patients [31].
Although fibrosis may evolve more slowly in African Americans, the rate of hepatocellular carcinoma is increasing more quickly in this population. Compared with non-Hispanic white men, among whom the incidence of hepatocellular carcinoma increased from 2.3 to 2.8 cases per 100,000 persons (for 19811985 vs. 19911995), the age-adjusted incidence among African American men increased from 5.3 to 6.1 cases per 100,000 persons in the same time period [32].
Not only is the rate of hepatocellular carcinoma among African American persons 2-fold higher than the rate among white persons [33], but the rate of liver cancerrelated mortality is 23 times higher among African American patients than among white patients [32]. More recent data confirm that the risk of hepatocellular carcinoma is twice as high among African American men than it is among white men [34].
TREATMENT
Although the prevalence of chronic HCV is higher in the African American population than in the white population, African American subjects are usually underrepresented in clinical trials. Moreover, despite improvements in antiviral therapy, rates of sustained response to treatment among African American patients are relatively poor (figure 1).
Figure 1. Progressive improvement in rates of sustained response to therapy for chronic hepatitis C virus infection in white and African American (AA) subjects. PegIFN, pegylated IFN; R, ribavirin.
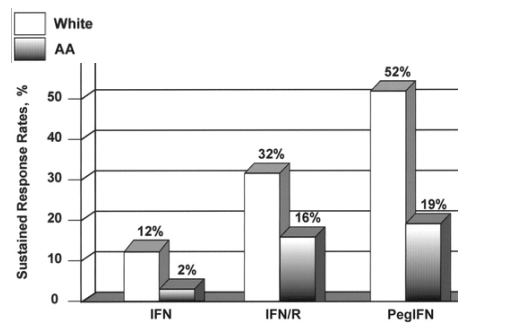
Older therapies. Several authors have reported inferior response rates in chronically HCV-infected African American patients who have received standard or consensus IFN therapy, with or without ribavirin (table 5).
Therapy today. In trials of pegylated IFN monotherapy treatment, there were inadequate numbers of African American subjects to make meaningful conclusions about the role of race in treatment outcomes [40, 41]. Likewise, in the registration trials of treatment with pegylated IFN and ribavirin, too few African American subjects were enrolled to make outcome assessments [42, 43].
Fortunately, 2 recent prospective trials have examined the effect of pegylated IFN treatment in a large number of African American subjects. Not only did these studies use pegylated IFN with ribavirintoday's standard of carebut they also enrolled the largest number of HCV-infected African American patients of any other trial to date. Both of these studies will be analyzed in detail.
The first trial [44] compared a group of 100 African American patients with a control group of 100 non-Hispanic white patients, both of which were treated with pegylated IFN-a-2b (1.5 g/kg per week) and ribavirin (1000 mg per day for 3 months, followed by 800 mg per day until week 48). Both groups were treatment naive and were equally matched with regard to infecting genotype, with each group including 98 genotype 1infected patients. The groups were also well matched with respect to age, duration of infection, viral load, alanine aminotransferase level, education level, and the presence of steatosis, fibrosis, and cirrhosis.
Treatment was well tolerated; 81% of African American and 79% of white patients completed therapy. Rates of adherence to treatment and of adverse events were also similar in both groups, and depression was the most common reason for discontinuation of therapy, regardless of ethnicity. Twenty-four percent of white patients and 22% of African American patients required dose reductions, with similar numbers of patients having the doses reduced because of neutropenia and anemia.
Compared with non-Hispanic white subjects, African American subjects had substantially poorer rates of sustained virologic response (19% vs. 52%; P < .001). The only predictor of sustained virologic response in multivariate analysis was race. Also analyzed was the predictive value of an early virologic response, defined as a 2-log10 reduction in HCV RNA level at week 12 of therapy. None of the subjects who did not achieve an early virologic response at week 12 reached a sustained virologic response, irrespective of ethnicity. Thus, the negative predictive value of early virologic response was 100%. However, fewer African American patients than white patients achieved an early virologic response (40% vs. 69%; P < .001). Moreover, there was a discrepant positive predictive value for patients who did achieve early virologic response (48 [83%] of 58 white patients with an early virologic response had a sustained virologic response, compared with only 18 [64%] of 28 such African American patients).
Study limitations included differences in sex, body weight, and diabetes status, some of which may have impacted response rates. Furthermore, alcohol use and abstinence were not documented in the study. Because alcohol affects response to IFN-based therapy [45], discrepancies in the 2 groups' rates of alcohol consumption may have influenced results. Finally, the ribavirin doses used were less than those customarily used for HCV genotype 1infected patients. Although this may have impacted rates of sustained virologic response, it would have not engendered any outcome differences between ethnic groups.
The second study [46] was a prospective, multicenter trial that enrolled 78 African American subjects and 28 white subjects who were infected with HCV genotype 1 and were treatment naive. All subjects received pegylated IFN-a-2a (180 g per week) and ribavirin (1000-1200 mg per day, dosed on the basis of weight) for 48 weeks. Unlike the study mentioned above, this trial allowed the use of growth factors. Sustained virologic response was the primary end point, but early virologic response was assessed at week 12 of the study. Pre- and posttreatment liver biopsy specimens (for 69 patients) were also evaluated for histologic improvement.
Patient characteristics, including age, viral load (high), alanine aminotransferase level, fibrosis score, and rate of cirrhosis, were similar in the 2 groups. However, there were discrepancies in the percentages of male participants and in mean body weight.
Rates of adverse events, especially injection site reactions, vomiting, alopecia, and xerosis, were higher among white patients. Thirty-nine percent of white subjects discontinued therapy, compared with 23% of African American subjects. Severe neutropenia (neutrophil count, <0.5 �~ 109 cells/L) occurred more frequently among African American subjects, and more patients in this group had their IFN treatment dose reduced for this reason (37% vs. 18%).
At week 72, in the African American group, the rate of sustained virologic response was 26% (95% CI, 16%-35%), which was significantly lower than the rate for the white group (39%; 95% CI, 21%-57%). The rate of sustained virologic response for white subjects was somewhat lower than that seen in registration trials [42, 43], but the authors attribute this anomaly to the high rate of premature discontinuation of therapy and to the small size of the cohort of patients. Significant predictors of sustained virologic response in multivariate analysis were age of <41 years, low pretreatment viral load, and an alanine aminotransferase level of <3 times the upper limit of normal.
With regard to histologic response, examination of paired biopsy specimens revealed improvement in fibrosis scores in 25% of African American patients. More than 90% of patients whose paired biopsy specimens were examined in both groups showed improvement or at least stabilization of fibrosis. Of the 36 African American patients who did not achieve sustained virologic response and who underwent both biopsies, 22% achieved fibrosis improvement. These data may support the concept that some patients may achieve reversal in fibrosis, irrespective of whether they achieved a sustained virologic response [47].
Study limitations included the high rate of treatment discontinuation in the white group, the lower rate of sustained virologic response in the white group, and higher weights in the African American group, which may have contributed to the poor response to IFN. Nonetheless, multivariate analysis failed to show a significant association between body mass index and nonresponse to treatment. Another limitation of the study was that racial disparity in histological outcomes did not reach statistical significance, because too few of the patients underwent paired liver biopsies. Like the previous study, this study did not indicate differences in alcohol use between patient groups.
Despite some limitations of the aforementioned studies, some important conclusions can be drawn from both. African American persons have lower rates of sustained virologic response to pegylated IFN combination therapy than do white persons, even when controlling for genotype 1 infection. Furthermore, the negative predictive value of not achieving an early virologic response at week 12 is reliable for both races. Despite the fact that early virologic response had an inferior positive predictive value for African American patients, patients of both ethnicities should be treated for 48 weeks if early virologic response is achieved.
Preliminary findings from the weight-based dosing of Peg-Intron and Rebetrol (WIN-R) trial demonstrate that weight-based dosing of ribavirin confers a significant advantage in the treatment of African American persons infected with genotype 1, compared with fixed dosing of ribavirin [25]. WIN-R is a prospective trial of 5000 treatment-naive HCV-infected patients from >200 US study centers designed to study weight-based versus fixed-dose ribavirin therapy. Patients were randomized to receive either pegylated IFN-a-2b, 1.5 ug/kg per week, plus ribavirin, 800 mg per day, or the same amount of pegylated IFN-a-2b plus ribavirin, 800-1400 mg per day, depending on weight. Baseline characteristics in the study's 2 arms were not significantly different. Three hundred eight-seven genotype 1infected African American patients were among those treated. Sixty-four percent of the African American patients had high viral loads, and 31% had at least bridging fibrosis (determined by biopsy), which is significantly more advanced disease than has been seen in previous trials. Erythropoietin or ribavirin reduction was permitted.
Although dose reductions occurred more frequently for patients who received weight-based doses of ribavirin than for those who received standard doses (12% vs. 8%), the rate of discontinuations of treatment for adverse events was not significantly different (18% vs. 17%). Anemia was no more common among patients who received 1400 mg of ribavirin per day than among those who received lesser doses. Of the 362 African American subjects who weighed >65 kg, those who received weight-based ribavirin dosing had better end-of-treatment and sustained virologic response rates than did those who received flat dosing; in fact, rates of sustained virologic response were more than doubled (sustained virologic response rate, 21% vs. 10%; P = .004). However, even though African American patients achieved higher response rates with weight-based doses of ribavirin, rates of sustained virologic response were still inferior to the rates for white patients.
A summary of the large treatment trials of African American persons who received pegylated IFN and ribavirin is shown in table 6.
Mechanisms for inferior treatment response.
A multitude of hypotheses have been promulgated to explain the dissimilar treatment responses among ethnic groups. As discussed previously, dysregulated CD4 function has been described in African Americans [29]. Other possibilities include discrepant viral kinetics, cytokine production, and iron stores.
An initial decrease in the HCV RNA level, referred to as "phase 1," occurs hours after the administration of IFN; it represents blocking of viral replication. The subsequent, slower decrease in the viral level (phase 2) represents the clearance of HCV-infected hepatocytes and usually occurs days to months after IFN therapy is initiated. The phase 2 decrease is the better predictor of ultimate HCV RNA clearance [48, 49]. Ethnicity may influence these phases. In a kinetics study that compared African American and white subjects who received combination therapy, the former had both small phase 1 and phase 2 decreases in the viral load [50]. A significant difference was found between African American and white subjects with regard to inhibition of viral production on the first day of treatment. The findings for phase 2 decreases in the viral load were also discordant; the rate of loss of infected cells was lower in African American subjects. The authors believed that the inadequate phase 1 decrease among African American patients accounted for the limited phase 2 decrease; thus, their poor response to therapy may be related to an impaired ability to block viral production early in treatment. However, when controlled for treatment effectiveness, differences in the decrease in the viral load were not statistically significant. More studies are clearly needed.
Ethnicity-associated cytokine production may also explain dissimilar treatment responses. A study compared cytokine production in phytohemaglutinin-stimulated PBMCs obtained from infected and control participants, both African American and white. Relative to healthy white control subjects, African American subjects produced higher levels of proinflammatory (TH1) cytokines IL-2 and TNF-a and lower levels of down-regulatory (TH2) cytokine IL-10. Furthermore, HCV-infected white patients who responded to treatment produced less IL-10 and more transforming growth factor-beta than did white subjects who did not respond to treatment. Because there were no African American patients who responded to therapy in this study, cytokine profiles could not be correlated with therapeutic outcome in this population. The authors postulated that the "subnormal" cytokine production among white responders may be more "permissive" to IFN-based therapy, as well as that the relatively more robust immune response among African American patients may yield inferior treatment results [51].
Elevated hepatic iron stores have also been invoked to explain resistance to IFN-based therapy in African Americans. Ioannou et al. [52] found that the risk of having increased iron stores, defined as elevated serum ferritin and transferrin saturation, was >5 times greater among HCV RNA-positive African American subjects than among HCV RNA-positive non-African American subjects. After adjustment for age, alcohol intake, sex, body mass index, and education level, HCV-positive African American patients with elevated aminotransferase levels had higher iron stores than did white patients. Because the response to standard IFN monotherapy may be influenced by hepatic iron content [53-55], the authors surmised that the discrepant iron stores may mirror discrepant treatment results. Limitations of the study include the assumptions that response rates to combination therapy with pegylated IFN and ribavirin are limited in the face of excess iron and that peripheral iron studies accurately reflect hepatic iron content.
Neutropenia.
African American persons have significantly lower mean concentrations of leukocytes and neutrophils than do white persons [56]. Lower neutrophil counts before commencement of treatment may lead to a greater likelihood that the IFN dose will be reduced during treatment or even that the patient will be excluded from participation in clinical trials.
In a multivariate analysis of a National Institutes of Health treatment study of 119 patients with chronic HCV infection who received standard IFN combination therapy, only African American race was associated with baseline neutropenia. Unlike prior treatment trials, neutropenia was not used as an exclusion criterion for therapy. Although African Americans have a >2-fold chance of developing neutropenia during treatment, none of the neutropenic patients developed serious bacterial infections. Furthermore, those with neutropenia had minimal additional cell count decrements during treatment. Thus, the authors recommended that constitutional (benign) neutropenia should not be an exclusion criterion for IFN-based therapy [57]. Similar results were evident in the preliminary analysis of the WIN-R trial [25]. Some authorities have even suggested lowering neutrophil thresholds for dose modifications in African American patients [58].
Finally, in the 2 large treatment trials of African Americans [44, 46], severe neutropenia was not associated with serious infection. However, in one study [46], neutropenia was the most common reason for modification of the IFN dose among African American patients (for 37% in this group). In the second trial [44], both races had similar rates of neutropenia episodes (13%14%).
HCV-HIV coinfection.
Approximately one-third of all HIV-infected persons in the United States are coinfected with HCV. One hundred eighty HCV/HIV-coinfected patients in an inner city area were evaluated for suitability for combination therapy with IFN plus ribavirin. African Americans were more than twice as likely to be ineligible than eligible for HCV treatment. Concomitant medical problems, psychiatric problems, poor adherence to treatment, and/or ongoing substance abuse were factors that led to patients being ineligible for therapy [59].
Of the 3 recent large trials that have focused on HCV treatment in HCV-HIV-coinfected persons, only 2 enrolled a significant percentage of African American subjects. However, race was not predictive for sustained response in either univariate or multivariate analyses in both studies [60, 61].
CONCLUSIONS
A summary of key differences between HCV-infected African American patients and white patients is shown in table 7. Despite lower rates of sustained response to contemporary therapy, therapeutic nihilism is not warranted when treating HCV-infected African American patients. With few exceptions, the African American population has been underrepresented in clinical trials of HCV infection, despite having a higher rate of infection; clearly, more clinical studies are needed, particularly those that investigate the mechanisms for disparate treatment responses. Likewise, prospective studies of African American patients are needed to clarify rates of disease progression in cases of chronic HCV infection. Later in 2006, final results are expected from a multicenter study sponsored by the National Institute of Diabetes and Digestive and Kidney Disease. This trial, called the Viral Resistance to Antiviral Therapy for Chronic Hepatitis C (VIRAHEP-C), has enrolled about 200 African American subjects and about 200 white subjects who will be treated with pegylated IFN and ribavirin. The purposes of the study are to assess response rates to therapy, as well as to analyze both viral factors and host factors, including genetic and immunologic variables that may influence treatment results.
|
|
| |
| |
|
|
|