| |
Study finds steatosis (fatty liver) is more common & severe in HIV coinfected compared to HCV monoinfected
|
| |
| |
"Impact of HIV on the prevalence and severity of steatosis in patients with chronic HCV"
Journal of Hepatology June 2006 pages 1026-1032
".....steatosis was significantly more common and more severe in HIV/HCV coinfected patients than in those with HCV monoinfection. Since steatosis is closely linked to the development and progression of fibrosis, our findings may help explain why patients with HIV/HCV coinfection have an accelerated rate of progression towards cirrhosis, an increased risk of end-stage liver disease, and a high incidence of liver-related death. These findings have important implications for the estimated 300,000 coinfected patients in the United States... If steatosis is a direct cytopathic effect of the HCV virus, it is possible that HIV leads to formation of steatosis simply by facilitating HCV replication.... Alternatively, it is possible that HIV acts synergistically with HCV, causing steatosis by disruption of lipid metabolism...."
Authors: Irphan Gaslightwala, Edmund J. Bini
Division of Gastroenterology, VA New York Harbor Healthcare System, 423 East 23rd Street, New York, NY 10010, USA
".....HIV/HCV coinfected patients were more likely to be black and also had a significantly lower BMI. In addition, coinfected patients were more likely to have a prior history of injection drug use, and had higher HCV RNA levels, higher serum triglyceride levels, higher total cholesterol levels, higher serum bilirubin levels, lower HDL cholesterol levels, and lower serum albumin levels....
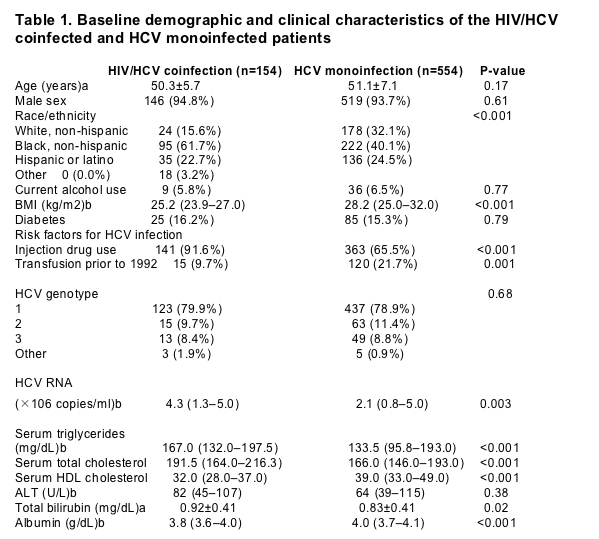
Steatosis of any grade was significantly more common in HIV/HCV coinfected patients than in HCV monoinfected subjects (72.1 vs. 52.0%, P<0.001), a difference of 20.1% (95% CI, 11.8-28.4%). The grade of steatosis was significantly more severe in HIV/HCV coinfected patients than in those with HCV monoinfection (Fig. 1). In addition, HIV/HCV coinfected patients were significantly more likely to have grade 2 or 3 steatosis (48.1 vs. 20.2%, P<0.001) and had a higher necroinflammatory score (6.8±2.6 vs. 5.9±2.5, P=0.009) as compared with HCV monoinfected subjects..... This association remained highly significant even after adjusting for baseline demographic and clinical characteristics, the grade of inflammation, and stage of fibrosis.
....In the univariate analysis, hepatic steatosis was found to be associated with obesity, diabetes, hypertriglyceridemia, low CD4 cell counts, and duration of antiretroviral therapy of ≥4 years. Although, steatosis was more common in patients infected with HCV genotype 3 and those with undetectable HIV viral loads, these differences were not statistically significant. In addition, we did not find any association between steatosis and the use of nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or any individual type of antiretroviral medication.
In the multivariable analysis, only hypertriglyceridemia (OR=6.69; 95% CI, 1.94-23.14) and antiretroviral therapy use for >/=4 years (OR=1.35)...
.... In addition to having more severe necroinflammation and steatosis, HIV/HCV coinfected patients were more likely to have advanced fibrosis..
.... Overall, the median fibrosis progression rate was 0.133 units/year (0.050-0.200 units/year) in the 143 coinfected patients and 0.071 units/year (0.037-0.111 units/year) in the 445 HCV monoinfected subjects in whom the duration of HCV infection could be determined....
ABSTRACT
Background/Aims
Although steatosis is strongly associated with hepatitis C virus (HCV) infection, little is known about this finding in patients coinfected with human immunodeficiency virus (HIV) and HCV. The aims of the present study were to determine the prevalence and severity of steatosis in HIV/HCV coinfected patients.
Methods
Consecutive patients undergoing liver biopsy were prospectively identified and were interviewed to obtain detailed demographic and clinical data. Steatosis was scored according to the percentage of hepatocytes involved: 0 (none), 1 (<33%), 2 (33-66%), or 3 (>66%); fibrosis was scored on a scale from 0 to 4.
Results
A total of 708 patients were enrolled, including 154 with HIV/HCV coinfection and 554 with HCV monoinfection.
Steatosis of any grade (72.1 vs. 52.0%, P<0.001), grade 2/3 steatosis (48.1 vs. 20.2%, P<0.001), and stage 3/4 fibrosis (43.5 vs. 30.0%, P=0.002) were significantly more common in coinfected patients.
Compared to HCV monoinfected subjects, HIV/HCV coinfection was associated with a significantly increased odds of steatosis of any grade (OR=3.21; 95% CI, 1.84-5.60) and grade 2/3 steatosis (OR=5.63; 95% CI, 3.05-10.36) after adjusting for potential confounding variables.
Among coinfected patients, the fibrosis progression rate increased in a linear fashion with the grade of steatosis.
Conclusions
Steatosis is more common and more severe in HIV/HCV coinfected patients than in those with HCV monoinfection.
1. Introduction
Steatosis is a common and important histologic finding in patients with chronic hepatitis C virus (HCV) infection, helping to identify those patients at risk for disease progression [1,2] or those who may have a poor response to antiviral therapy [3]. Previous studies have demonstrated associations between the severity of steatosis and infection with genotype 3 [4-7], obesity [5,8,9], and diabetes [10]. It is likely that the etiology of steatosis in patients with HCV monoinfection is multifactorial, related to virus-induced disruption of lipid metabolism as well as host factors that promote steatogenesis [11-14].
It is estimated that HCV affects approximately 15-30% of patients with human immunodeficiency virus (HIV) infection, although the prevalence varies widely depending on the population studied [15,16]. Patients with HIV/HCV coinfection have an accelerated course of liver disease [17,18], and end-stage liver disease is a leading cause of mortality in this population [19,20]. Steatosis may occur in HIV infection as a result of the HIV virus itself, or may be secondary to coinfection with HCV, antiretroviral medication use, comorbid diabetes, alcohol intake, obesity, or other factors [21,22].
Despite the clinical importance of steatosis and the high-burden of disease resulting from HIV/HCV coinfection, little is known about steatosis formation or the risk factors for steatosis in this population. The aims of the present study were to determine the prevalence and severity of steatosis in patients coinfected with HIV/HCV, and to compare this to a group of patients with HCV monoinfection. In addition, we sought to evaluate factors associated with steatosis among coinfected subjects. We hypothesized that steatosis is more common and more severe in patients with HIV/HCV coinfection than in those with HCV monoinfection.
2. Methods
2.1. Study population
We prospectively identified all patients with HIV/HCV coinfection or HCV monoinfection who were referred for liver biopsy prior to HCV treatment at the Veterans Affairs (VA) New York Harbor Healthcare System in New York City between September 1998 and July 2004. Patients were eligible for this study if they were 18 years of age or older, were seropositive for HCV antibody (Ortho HCV ELISA version 3.0; Ortho-Clinical Diagnostics, Inc., Raritan, NJ), and had detectable HCV RNA (COBAS Amplicor HCV Monitor Test version 2.0, Roche Molecular Systems, Branchburg, NJ).
Patients were excluded from this study if they were coinfected with hepatitis B, had other known causes of liver disease, consumed more than two drinks of alcohol per day, were previously treated for HCV, or if they had decompensated cirrhosis (ascites, hepatic encephalopathy, or esophageal or gastric varices). All patients provided written informed consent, and the study was approved by the Institutional Review Board at our medical center.
2.2. Study design
Prior to liver biopsy, all patients were interviewed by a trained research assistant to obtain detailed demographic and clinical information. Data collected on each patient included age, sex, race/ethnicity, medical and other comorbid conditions, self-reported use of alcohol, risk factors for HIV and HCV, duration of HIV and HCV infection, and medication use.
A comprehensive physical examination was performed, including measurement of height and weight. Laboratory testing performed on each subject prior to liver biopsy included complete blood count, serum electrolytes, liver profile, prothrombin time, fasting triglycerides, serum total and high-density lipoprotein (HDL) cholesterol, HCV RNA testing, and HCV genotyping (Inno-LiPA HCV II assay, Innogenetics, Gent, Belgium). In addition, CD4 lymphocyte counts and plasma HIV RNA testing (VERSANT HIV-1 RNA 3.0 Assay [bDNA], Bayer Diagnostics, Tarrytown, NY) were performed on all HIV seropositive subjects.
Percutaneous liver biopsies were performed using a Menghini needle. Biopsy specimens were fixed in formalin, embedded in paraffin, and stained with hematoxylin-eosin and Masson trichrome stains. Biopsy specimens were evaluated by a board-certified staff pathologist who was blinded to the aims of this study, although the pathologist was not blinded to the HCV or HIV status of the patients. Using the classification system for steatohepatitis proposed by Brunt et al. [23], steatosis was graded as 0 (none), 1 (<33% of hepatocytes), 2 (33-66% of hepatocytes), or 3 (>66% of hepatocytes). Necroinflammatory activity was scored from 0 to 18 using the Ishak grading system [24], whereas fibrosis was scored on a scale from 0 to 4 using the Scheuer staging system [25].
2.3. Study outcomes
The primary outcome measure was the proportion of patients with steatosis on liver biopsy. For the primary analysis, patients were considered to have steatosis if they had steatosis of any grade (grade 1, 2, or 3) on liver biopsy. The proportion of patients who had steatosis on liver biopsy was compared between HIV/HCV coinfected patients and subjects with HCV monoinfection.
The secondary outcomes of this study included the severity of steatosis and the type of steatosis identified (macrosteatosis, microsteatosis, or mixed) in HIV/HCV coinfected and HCV monoinfected subjects. In addition, we evaluated factors associated with steatosis among coinfected patients, as well as the relationship between steatosis and fibrosis progression among coinfected and HCV monoinfected patients. In patients with an identifiable risk factor for HCV infection, fibrosis progression rates were calculated by dividing the stage of fibrosis by the duration of HCV infection. The duration of HCV infection was estimated from the date of first exposure to injection drug use or blood transfusion. Fibrosis progression rates are expressed as units of fibrosis per year.
2.4. Statistical analysis
Continuous variables were compared using an unpaired t-test, the Mann-Whitney U-test, or the Kruskal-Wallis test as appropriate. Data are expressed as means±SD for those variables that were normally distributed, and medians and interquartile range (25-75th percentile) for those with a non-normal distribution. Categorical variables are expressed as proportions and were compared using the chi-square test or Fisher's exact test. Spearman rank correlations were used to assess the association between the grade of steatosis and the stage of fibrosis.
Univariate logistic regression analysis was utilized to identify factors associated with presence of steatosis. For continuous predictors, the variables were converted to dichotomous values using the median or clinically relevant cutoff points. Subsequently, a multivariable logistic regression model was created involving all significant variables identified in the univariate analyses. The strength of the association between covariates and presence of steatosis is expressed as odds ratios (OR) with 95% confidence intervals (CI). Statistical analysis was performed using SPSS software version 13.0 for Windows (SPSS, Inc., Chicago, IL) and a two-tailed P-value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of the study subjects
A total of 708 patients met the eligibility criteria and were enrolled in the study. The baseline characteristics of the 154 HIV/HCV coinfected patients and the 554 HCV monoinfected subjects are shown in Table 1. Although there were no differences in age or sex between the two groups, HIV/HCV coinfected patients were more likely to be black and also had a significantly lower BMI. In addition, coinfected patients were more likely to have a prior history of injection drug use, and had higher HCV RNA levels, higher serum triglyceride levels, higher total cholesterol levels, higher serum bilirubin levels, lower HDL cholesterol levels, and lower serum albumin levels.
Among the HIV/HCV coinfected patients, the median duration of HCV infection was 20.0 years (13.0-22.0 years) and the median duration of HIV infection was 7.5 years (4.0-12.0 years). Patients had a median CD4 count of 429cells/mm3 (209-547cells/mm3), with 31 (20.1%) having a CD4 count below 200cells/mm3. A total of 130 (84.4%) were taking antiretroviral therapy, and the median duration of use was 4.0 years (3.0-10.0 years). Plasma HIV RNA was undetectable in 78 (50.6%) of the 154 coinfected subjects.
3.2. Prevalence and severity of steatosis
The median size of the liver biopsy specimens obtained was 17.0mm (15.0-25.0mm) in HIV/HCV coinfected patients and 18.0mm (13.0-24.0mm) in HCV monoinfected subjects (P=0.86).
Steatosis of any grade was significantly more common in HIV/HCV coinfected patients than in HCV monoinfected subjects (72.1 vs. 52.0%, P<0.001), a difference of 20.1% (95% CI, 11.8-28.4%). The grade of steatosis was significantly more severe in HIV/HCV coinfected patients than in those with HCV monoinfection (Fig. 1). In addition, HIV/HCV coinfected patients were significantly more likely to have grade 2 or 3 steatosis (48.1 vs. 20.2%, P<0.001) and had a higher necroinflammatory score (6.8±2.6 vs. 5.9±2.5, P=0.009) as compared with HCV monoinfected subjects.
To determine the strength of the association between HIV/HCV coinfection and the presence of steatosis, we analyzed all 708 patients using multivariable logistic regression analysis. Compared to HCV monoinfected subjects, HIV/HCV coinfection was associated with a significantly increased odds of steatosis of any grade as well as grade 2/3 steatosis (Table 2). This association remained highly significant even after adjusting for baseline demographic and clinical characteristics, the grade of inflammation, and stage of fibrosis.
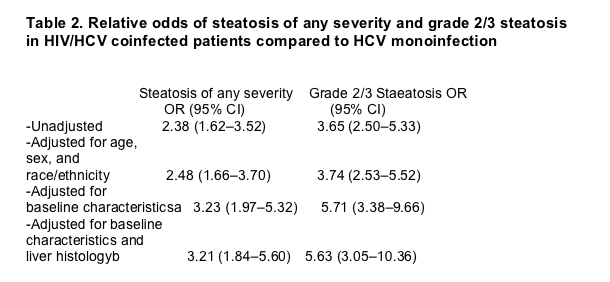
Among the 111 HIV/HCV coinfected patients and the 288 subjects with HCV monoinfection who had steatosis of any grade on liver biopsy, we found that the type of steatosis differed significantly between the two groups (Fig. 2). Although the majority of patients with HCV monoinfection had macrosteatosis, patients with coinfection were more likely to have a mixed pattern of steatosis (macrosteatosis and microsteatosis) or microsteatosis alone.
Fig. 2. Type of steatosis in the 111 HIV/HCV coinfected and the 288 HCV monoinfected patients who had steatosis of any grade on liver biopsy. The type of steatosis differed significantly between HIV/HCV coinfected patients and those with HCV monoinfection (P<0.001), with mixed steatosis (macrosteatosis+microsteatosis) and microsteatosis being more common in coinfected patients.
The prevalence of microsteatosis among the 154 HIV/HCV coinfected patients was significantly higher among patients taking any type of antiretroviral medication (15.4 vs. 0.0%, P=0.04) and those taking nucleoside reverse transcriptase inhibitors (15.4 vs. 0.0%, P=0.04) as compared with subjects who were not taking these medications. However, microsteatosis was not more common in patients taking non-nucleoside reverse transcriptase inhibitors (15.9 vs. 11.0%, P=0.38) or protease inhibitors (13.2 vs. 12.8%, P=0.95).
3.3. Predictors of steatosis in HIV/HCV coinfected patients
To evaluate factors associated with steatosis among the 154 HIV/HCV coinfected patients, we determined the proportion of patients who had steatosis of any grade on liver biopsy according to select demographic and clinical characteristics (Table 3). In the univariate analysis, hepatic steatosis was found to be associated with obesity, diabetes, hypertriglyceridemia, low CD4 cell counts, and duration of antiretroviral therapy of ≥4 years. Although, steatosis was more common in patients infected with HCV genotype 3 and those with undetectable HIV viral loads, these differences were not statistically significant. In addition, we did not find any association between steatosis and the use of nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or any individual type of antiretroviral medication.
In the multivariable analysis, only hypertriglyceridemia (OR=6.69; 95% CI, 1.94-23.14) and antiretroviral therapy use for >/=4 years (OR=1.35; 95% CI, 1.18-1.55) were found to be independent predictors of steatosis. Higher triglyceride levels and a longer duration of antiretroviral therapy use remained independent predictors of steatosis even when the multivariable logistic regression model was repeated using continuous instead of dichotomous variables. These two predictors also remained significant when age, sex, and race/ethnicity were forced into the model or when the model included the grade of inflammation and stage of fibrosis.
3.4. Relationship between steatosis and fibrosis
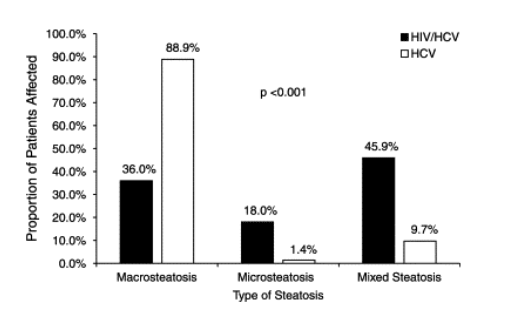
In addition to having more severe necroinflammation and steatosis, HIV/HCV coinfected patients were more likely to have advanced fibrosis (Fig. 3). Stage 3 or 4 fibrosis was present in 43.5% of coinfected patients as compared with 30.0% of HCV monoinfected subjects (P=0.002).
Fig. 3. Severity of fibrosis in the 154 HIV/HCV coinfected and 554 HCV monoinfected patients. The stage of fibrosis differed significantly between HIV/HCV coinfected patients and those with HCV monoinfection (P=0.003).
Using Spearman correlation analysis, we found that the grade of steatosis was strongly correlated with the stage of fibrosis in the 154 coinfected patients (r=+0.49, P<0.001) and in the 554 HCV monoinfected subjects (r=+0.56, P<0.001). The prevalence of steatosis was significantly higher in coinfected patients (83.6 vs. 63.2%, P=0.005) and monoinfected subjects (82.5 vs. 38.9%, P<0.001) with grade 3 or 4 fibrosis than in those with less advanced disease.
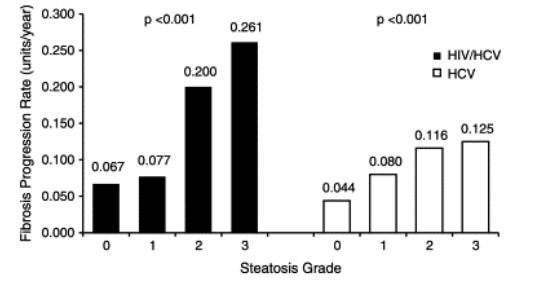
Overall, the median fibrosis progression rate was 0.133 units/year (0.050-0.200 units/year) in the 143 coinfected patients and 0.071 units/year (0.037-0.111 units/year) in the 445 HCV monoinfected subjects in whom the duration of HCV infection could be determined (P<0.001). The median fibrosis rate was 0.154 units/year (IQR, 0.069-0.258 units/year) in coinfected patients with steatosis as compared with 0.067 units/year (0.001-0.150 units/year) in coinfected subjects without steatosis on liver biopsy (P<0.001). Moreover, the fibrosis progression rate increased in a linear fashion with the grade of steatosis among HIV/HCV coinfected patients as well as in HCV monoinfected subjects (Fig. 4).
Fig. 4. Median fibrosis progression rates in the HIV/HCV coinfected and HCV monoinfected patients stratified according to the severity of steatosis. Median fibrosis progression rates were calculated for the 143 coinfected and 445 HCV monoinfected patients with a known duration of HCV infection by dividing the stage of fibrosis by the duration of HCV infection. The fibrosis progression rate is expressed as units of fibrosis per year and increased significantly with the severity of steatosis in a linear fashion in both coinfected (P<0.001) and HCV monoinfected subjects (P<0.001).
4. Discussion
In the present study, we found that steatosis was significantly more common and more severe in HIV/HCV coinfected patients than in those with HCV monoinfection. Since steatosis is closely linked to the development and progression of fibrosis, our findings may help explain why patients with HIV/HCV coinfection have an accelerated rate of progression towards cirrhosis, an increased risk of end-stage liver disease, and a high incidence of liver-related death. These findings have important implications for the estimated 300,000 coinfected patients in the United States [16].
Numerous studies have evaluated the prevalence and severity of steatosis among patients with HCV monoinfection. In a recent review, Asselah et al. calculated the weighted mean prevalence of steatosis based on a pooled analysis of 3607 patients and found that steatosis was present in 54% of HCV infected subjects [26]. The prevalence of steatosis among the HCV monoinfected patients in our study (52.0%) is comparable to that reported in previous studies, lending further validity to our findings.
Although, a large number of studies have evaluated steatosis in patients with HCV monoinfection, little is known about this histologic finding in patients coinfected with HIV/HCV. A recent study by Sulkowski et al. [27] reported that steatosis was present in 40% of the 112 HIV/HCV coinfected patients randomly selected from the HIV clinic cohort at Johns Hopkins University. However, this study did not include a comparison group of HCV monoinfected subjects.
In another recent study, Monto et al. [28] found that steatosis was present in 47% of the 92 HIV/HCV coinfected subjects and 59% of those with HCV monoinfection. In contrast to the studies by Sulkowski et al. [27] and Monto et al. [28], we found a higher prevalence of steatosis in our coinfected patients (72.1%). More importantly, the increased odds of steatosis in coinfected patients remained highly significant even after adjusting for potential confounding variables using multivariable logistic regression analysis. The reasons for the different findings between our study and the previous two studies are unknown, but are likely due to differences in the patient populations studied.
There are several models to potentially explain how HIV interacts with HCV to promote steatogenesis. If steatosis is a direct cytopathic effect of the HCV virus, it is possible that HIV leads to formation of steatosis simply by facilitating HCV replication. Patients with HIV/HCV coinfection are known to have markedly increased HCV RNA levels within liver tissue [29], and higher levels of intrahepatic HCV RNA have been shown to correlate with the severity of steatosis in HCV monoinfected subjects [30,31].
Alternatively, it is possible that HIV acts synergistically with HCV, causing steatosis by disruption of lipid metabolism. It is interesting to note that hypertriglyceridemia was independently associated with steatosis in our coinfected patients, suggesting that this mechanism may have contributed to steatogenesis in our subjects.
In addition, antiretroviral therapy has clearly been implicated in development of steatosis in patients with HIV [21,22], and we found that a longer duration of antiretroviral therapy was independently associated with steatosis. Nucleoside analogues may interfere with mitochondrial function, and have been associated with severe hepatic steatosis, lactic acidosis, and hepatic failure [32,33].
Steatosis associated with HCV monoinfection is typically macrovesicular, as is steatosis associated with HIV [21,34]. In our study, however, patients with HIV/HCV coinfection were more likely to have mixed pattern of macrovesicular and microvesicular steatosis. The reason for this finding is unclear, but it suggests that a different mechanism, or a multitude of mechanisms, may be responsible for steatosis formation in the coinfected population.
Another interesting and important finding of our study was the strong association between the steatosis and hepatic fibrosis in HIV/HCV coinfected patients. In our coinfected subjects, the fibrosis progression rate increased in a linear fashion with the severity of steatosis. The association between hepatic steatosis and fibrosis highlights the potential role of steatosis in the pathogenesis of fibrosis and cirrhosis in coinfected patients.
The strengths of our study include the prospective study design, collection of detailed demographic and clinical data, enrollment of a relatively large sample size, the racial/ethnic diversity of our patients, and inclusion of a control group of HCV monoinfected subjects. However, several limitations of our study deserve consideration. First, the majority of our patients were men and all were enrolled at a single VA medical center. Therefore, our findings may not be generalizable to women or to non-VA settings. The preponderance of men in our study may affect the observed prevalence of steatosis. Non-alcoholic fatty liver disease may have a gender bias [35], and it is possible that gender may similarly influence the prevalence of steatosis in HIV/HCV coinfection or HCV monoinfection.
Second, our study included subjects who were referred for diagnostic liver biopsy and, therefore, the prevalence of steatosis in our subjects may not be representative of all patients with HIV/HCV coinfection. Finally, although we clearly demonstrated that coinfected patients had a high prevalence of steatosis on liver biopsy, the cross-sectional design of our study does not allow us to prove causality. This important limitation underscores the need for well-designed studies with paired liver biopsies to evaluate the natural history of steatosis and fibrosis in HIV/HCV coinfected patients.
In conclusion, we have shown that steatosis is highly prevalent and more severe in patients coinfected with HIV/HCV than in HCV monoinfected subjects. The increased prevalence and severity of steatosis in the coinfected population offers a potential mechanism for the more rapid rate of fibrosis progression in these patients. Further studies are needed to confirm our findings and to clarify the pathogenesis and impact of steatosis in HIV/HCV coinfection.
|
|
| |
| |
|
|
|