| |
Peginterferon alfa-2a (Pegasys) and ribavirin for chronic hepatitis C genotype 1 infections in black patients: safety, tolerability and impact on sustained virologic response
|
| |
| |
....SVR rate in blacks was related to patient tolerance and degree of adherence to treatment (33% is best SVR rate when adherence is >80% in this study suggesting that with 100% adherence SVR rates would increase)..... these results suggest that black American patients can be treated safely with pegylated interferons plus ribavirin. Our results suggest that the SVR might be improved in black patients by a greater focus on managing the side effects of peginterferon and ribavirin and by increasing patient adherence to treatment. In addition, the lack of association between constitutional and peginterferon-induced neutropenia and infectious AEs suggest a lower ANC threshold for peginterferon dose reductions might be safe..... New clinical studies are underway to test higher doses of peginterferon alfa-2a and ribavirin, longer courses of therapy, combinations with immunomodulators, and new antiviral drugs to improve virologic response rates.
Journal of Viral Hepatitis
Volume 13 Page 371 - June 2006
C. D. Howell1, L. S. Jeffers2, W. Cassidy3, K. R. Reddy4, S. Hu5 and J. S. Lee5
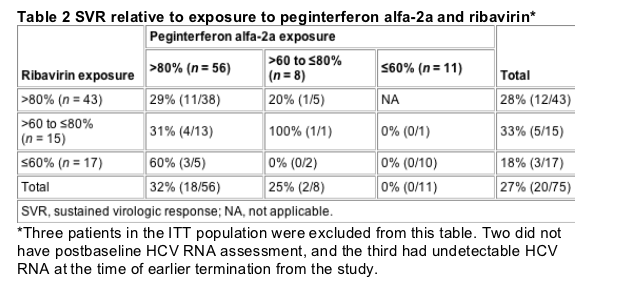
Fig. 2 Relationship between cumulative exposure to peginterferon alfa-2a and ribavirin (a) and peginterferon alfa-2a or ribavirin (b) and outcome in all black patients (n =75). The full planned dose of peginterferon alfa-2a per patient was 8640 mg (180 mg/week X 48 weeks), and the full planned dose of ribavirin per patient was 403 200 mg (1200/day X 336 days) for patients weighing ≥75 kg and 336 000 mg (1000/day X 336 days) for those weighing <75 kg.
Summary. HCV infections are two-times more prevalent in black Americans than in whites. We previously reported that treatment with peginterferon alfa-2a plus ribavirin produced a sustained virologic response (SVR) rate of 26% in blacks, a lower efficacy compared with the SVR in whites. Here we detail the safety profile of peginterferon alfa-2a plus ribavirin and the relationship between treatment adherence, defined by cumulative drug exposure, and SVR in 78 black patients infected with HCV genotype 1.
Sixty-two (79%) patients completed 48 weeks of combination treatment. Peginterferon alfa-2a dose was modified for neutropenia in 36 patients (46%), whereas ribavirin dose was modified due to anemia in 31 patients (40%).
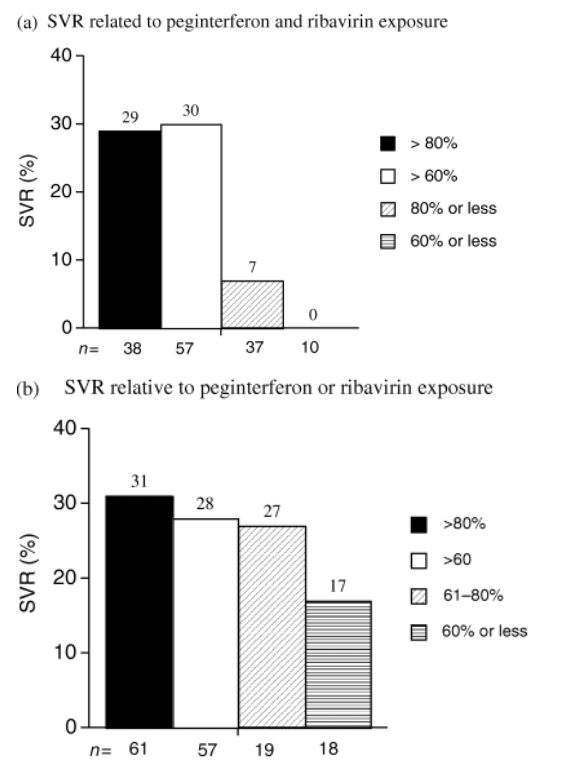
The SVR rate was related to medication exposure, based on the percentage of the planned doses of peginterferon and ribavirin that the patients received.
The SVR rates were 33, 25 and 0% in patients who received >80, 61-80 and ≦60% of the planned peginterferon, respectively.
The SVR rates were 28, 33 and 18% in patients who received >80, 61-80 and ≦60% of the planned ribavirin.
The SVR rate was 29% (11 of 38) in patients who received >80% of the total planned doses of both peginterferon and ribavirin and 7% (1 of 14) in patients who received ≦80% of both medicines.
The SVR was 30% in patients who received >60% exposure to both, and 0% in patients with ≦60% exposure.
In conclusion, peginterferon alfa-2a plus ribavirin demonstrated good safety and tolerability profiles in blacks infected with HCV genotype 1. Adherence to at least >60% of the planned peginterferon alfa-2a and ribavirin doses for 48 weeks was associated with a greater SVR in black patients with HCV genotype 1 infections.
Introduction
The prevalence of HCV infection in the United States varies among different racial groups. For example, the prevalence of antibodies to HCV and complications of chronic HCV infection, including hepatocellular carcinoma, are 2-3 times higher in blacks than in whites [1,2]. Treatment with pegylated interferons plus ribavirin has become the standard of care for patients infected with chronic hepatitis C [3]. In large international clinical trials, treatment with pegylated interferons plus ribavirin resulted in SVR rates of 54-63% [4-6]. Patients infected with HCV genotype 1, however, had somewhat lower SVR rates, ranging from 42 to 52% [4-6]. More recent clinical trials have found much lower sustained virologic response (SVR) rates in black American patients compared with white patients [4-8]. We recently reported that combination therapy with peginterferon alfa-2a plus ribavirin resulted in an SVR rate of 26% in 78 black Americans (intention to treat or ITT population) chronically infected with HCV genotype 1 [7]. Another study, using peginterferon alfa-2b plus ribavirin, found an SVR of 19% in blacks [8]. The causes of lower efficacy of these treatments in blacks are as yet unknown.
One potential obstacle to interferon-based therapies for HCV in black patients is the higher prevalence of constitutional neutropenia, as documented in several epidemiologic studies [9-11]. There is no evidence that constitutional neutropenia increases the incidence of serious infections. Yet, this remains a concern when contemplating treatment of HCV-infected blacks because interferon therapy is known to cause bone marrow suppression [9,11,12]. Indeed, baseline neutrophil counts below 1500/mL have excluded many blacks from participation in HCV clinical trials.
To determine the extent to which medication dose modifications contributed to the lower efficacy of combination treatment with peginterferon alfa-2a plus ribavirin, we studied the relationships between patient adherence to therapy and the SVR in the cohort of 78 black patients from our initial report [7]. We have focused primarily on the impact of this therapeutic regimen on hematological parameters.
METHODS
Study design
This was an open-label, multicenter study conducted in the United States to establish the efficacy of peginterferon alfa-2a plus ribavirin as initial treatment in non-Hispanic black patients chronically infected with HCV genotype 1 [7]. As described previously, a small reference group of non-Hispanic white patients was included to ensure that SVR rates were consistent with results reported in the registration trial of peginterferon alfa-2a plus ribavirin [5]. As such, the ratio of black patients to white patients was 3:1 [7]. Written informed consent was obtained from each patient, and the study protocol was reviewed and approved according to ethical guidelines of the 1975 Declaration of Helsinki by institutional review boards at the respective sites. The inclusion and exclusion criteria for this trial were similar to those established in the pivotal trials of peginterferon alfa-2a plus ribavirin [5,6].
Each patient received peginterferon alfa-2a 180 mg sc once weekly plus ribavirin 1000-1200 mg/day orally in split doses. Patients weighing <75 kg received 1000 mg/day of ribavirin (400 mg in the morning and 600 mg in the evening), while patients weighing ≥75 kg received 1200 mg/day of ribavirin (600 mg each in the morning and evening). The study protocol did not mandate or recommend that patients be discontinued from treatment for virologic non-response. Thus, study investigators were encouraged to continue patients on treatment even when serum HCV RNA was detected at week 24 of treatment. Patients were treated for 48 weeks and were followed for an additional 24 weeks. The primary clinical endpoint was the SVR, defined as an undetected serum HCV RNA (<50 IU/mL) by the Roche Cobas Amplicor HCV assay, Version 2.
Safety assessments
Safety was assessed by recording adverse events (AEs) reported by patients, clinical laboratory test results (hematology, blood chemistry, thyroid function tests and urinalysis) and vital signs. The safety population included all enrolled patients who received at least one dose of study medication and had at least one safety assessment after the baseline visit.
Dose reductions
Peginterferon alfa-2a and ribavirin doses were reduced for safety reasons according to predetermined criteria. Peginterferon dose was decreased to 135 mg/week when the absolute neutrophil count (ANC) fell below 1000 cells/mL, and to 90 mg/week for ANC below 750 cells/mL. Ribavirin was reduced from 1000 or 1200 to 600 mg/day if the haemoglobin level fell below 10 g/dL or if the patient experienced a fall in haemoglobin of >2 g/dL during any 4 weeks of treatment. Treatment with haematopoietic growth factor(s) was permissible for severe anaemia and neutropenia at the discretion of the investigators. Specifically, erythropoietin treatment could be initiated if haemoglobin concentration was reduced by >3 g/dL. Erythropoietin and G-CSF were administered to 3 (4%) black patients during treatment, as previously reported [7].
Protocol adherence criteria
To determine the impact of adherence to the peginterferon and ribavirin treatment, we compared the SVR in patients based on the cumulative drug exposure, defined as the total dose of each drug received relative to the planned dose of that drug over the 48-week treatment period. The full planned dose of peginterferon alfa-2a per patient was 8640 mg (180 mg/week X 48 weeks), and the full planned dose of ribavirin per patient was 403,200 mg (1200/day X 336 days) for patients weighing ≥75 kg and 336,000 mg (1000/day X 336 days) for those weighing <75 kg. Cumulative drug exposures was stratified into three categories: ≦60%, 61-80% and >80%. The rationale for these thresholds was that 10 of 11 patients with ≦60% exposure to both peginterferon alfa-2a and ribavirin failed to complete the 48-week treatment protocol. In contrast, 61 (95%) of 64 who received >60% of either peginterferon or ribavirin completed the 48-week treatment protocol.
Data analysis
Safety variables were analysed using proportions, frequency distributions, and descriptive statistics, as appropriate. The impact of peginterferon alfa-2a and ribavirin dose reductions and discontinuations on SVR was summarized using descriptive statistics.
RESULTS
A total of 78 black patients participated in the study. Sixty-two (79%) patients completed 48 weeks of treatment and 60 (77%) completed the 24-week post-treatment follow up. Using an ITT analysis, 20 black patients (26%; 95% CI, 16-35%) achieved a SVR [7].
Hematological parameters
Changes in haematological parameters were evaluated during the 72-week study period and were consistent with the known effects of peginterferon alfa-2a and ribavirin (Fig. 1). Compared with the white reference group, both the mean baseline ANC (2.9 ± 0.15 X 103/mL vs 3.5 ± 0.19 X 103/mL) and the ANC nadir (0.9 + 0.06 X 103/mL vs 1.2 ± 0.07 X 103/mL) were lower in the black patients (Table 1). There was a sharp decrease in median ANC during the first week of treatment for both the black (Fig. 1a) and white (data not shown) patients. The maximum reduction in ANC from baseline was 45% for the black group and 51% for the white group (Table 1), indicating similar degrees of granulocyte suppression. Significant neutropenia (ANC < 500 cells/mL) was observed in 31 of the 78 (40%) black patients, compared with 5 of the 28 (19%) white patients. None of these patients developed serious infections. In both blacks and whites, the ANC returned to baseline values within 3 months after the completion of therapy.
Median lymphocyte (Fig. 1b) and platelet (Fig. 1c) counts and median haemoglobin concentrations (Fig. 1d) showed trends similar to those observed for median ANC. There were no race-related differences in lymphopenia, anaemia and thrombocytopenia. Five of 78 (6%) black patients experienced a decrease in haemoglobin concentration to <8.5 g/dL, but no patient had ribavirin discontinued due to anaemia. Two black patients (3%) experienced a reduction in platelet counts to <50 X 103/mL, but there were no serious complications. Reductions in lymphocyte and platelet counts and haemoglobin concentration were also transient, returning to baseline values shortly after the completion of therapy.
Serious AEs
As reported previously, the incidences of most AEs in black patients were comparable to those in the pivotal trials for similar treatment arms across all body systems [6-8]. Seven black patients experienced serious AEs during the treatment period. No serious AE was considered related to treatment. Two infectious AEs occurred. One case of appendicitis at week 8 was considered possibly related to treatment with peginterferon alfa-2a plus ribavirin. A case of cellulitis on day 493 (22 weeks after treatment was completed) was judged to be unrelated to treatment. These serious infections were not associated with severe neutropenia or lymphocytopenia. One patient died during the study period, due to myocardial infarction on day 516 (week 74), but this death was considered unrelated to treatment.
Dose modifications
Peginterferon alfa-2a dose was modified (withheld or reduced) because of an AE or laboratory abnormality in 36 of 78 (46%) black patients; ribavirin dose was modified as the result of an AE or laboratory abnormality in 31 (40%) black patients. By comparison, peginterferon alfa-2a and ribavirin doses were reduced in 29 and 46% of whites [8]. The most common reason for modification of the peginterferon alfa-2a dose was neutropenia, occurring in 29 of 36 (81%) black patients who required dose reductions. Anaemia was the most frequent reason for modification of ribavirin dose, occurring in 19 of 31 (61%) patients. In most cases, dose reductions for both peginterferon alfa-2a and ribavirin were transient.
Eighteen (23%) black patients withdrew from the study prior to week 72. Of these, eight failed to return for follow up, four withdrew consent, and one was discontinued due to protocol violation. The study protocol did not mandate or recommend that treatment be discontinued even if the serum HCV RNA remained detected after 24 weeks. Thus, only one patient was discontinued due to insufficient therapeutic response. Four blacks (5%) were discontinued prematurely by the investigators for AEs or laboratory abnormalities, two for neutropenia and two for serious infections.
Protocol adherence and treatment outcome
For each patient, cumulative exposure to peginterferon alfa-2a and ribavirin was defined as the total dose of each medication received relative to the planned dose over the 48-week treatment period. There was a strong concordance between peginterferon and ribavirin exposure and adherence to 48 weeks of treatment. Fifty-one patients (68%) had >60% peginterferon exposure, and 49 (96%) completed the 48-week treatment protocol. Fifty-eight (77%) patients had >60% ribavirin exposure, and 55 (95%) completed 48 weeks of treatment. In contrast, 10 of 11 (91%) patients with ≦60% exposure to peginterferon and 14 of 19 (74%) patients with ≦60% ribavirin exposure discontinued treatment before week 48.
The SVR rates relative to cumulative peginterferon alfa-2a and/or ribavirin exposures of ≦60, 61-80 and >80% are shown in Table 2 and Fig. 2. The SVR rate was 32% in patients with >80% peginterferon exposure and 25% in patients with 61-80% exposure (Table 2, bottom row). No patient with ≦60% peginterferon exposure had a SVR. The SVR rate was 28% in patients with >80% ribavirin exposure, and 33% in patients with 61-80% exposure (Table 2, fifth column). Three patients (18%) with ≦60% ribavirin exposure had a SVR.
Figure 2a shows the SVR in black patients in whom cumulative peginterferon and ribavirin exposure were concordant. The SVR rate was 29% for patients with >80% exposure, and 30% for patients with >60% exposure to both peginterferon and ribavirin (Table 2; Fig. 2a). The SVR was 7% (1 of 14) for patients with ≦80% exposure and 0% for patients with ≦60% exposure to both medications. Due to variability in peginterferon and ribavirin dose reductions, many patients had discordant levels of peginterferon and ribavirin exposure. Figure 2b shows the SVR rates relative to levels either peginterferon or ribavirin exposure. The SVR was 31% when exposure to either medication was >80 and 28% when exposure was >60%. A similar SVR (27%) was found in patients with 61-80% exposure to either peginterferon or ribavirin. There was a progressive decrease in SVR with exposures <80 and <60% to either medicine. Seven of 9 (78%) SVR patients with <80% exposure to either medicine had >80% peginterferon exposure and 8 (89%) patients had <80% ribavirin exposure (Table 2). The three SVR patients with <60% exposure to either medication had >80% peginterferon exposure combined with <60% ribavirin exposure (Table 2).
DISCUSSION
We examined the relationships between patient adherence to therapy and the SVR in a cohort of 78 black HCV patients who were treated with peginterferon alfa-2a plus ribavirin treatment for 48 weeks. Peginterferon alfa-2a and ribavirin were safe and well-tolerated in black American HCV genotype 1 patients, with a safety and tolerability profiles similar to that reported in White patients in previous trials [5]. The SVR rate in blacks was related to patient tolerance and degree of adherence to treatment.
During this trial, no unexpected treatment related AEs were observed in blacks. Not surprisingly, neutropenia was frequent in black patients, with neutrophil counts below 0.75 X 103/mL occurring in 40% of blacks [7]. Neutropenia was the indication for 80% of peginterferon dose reductions. However, we found no relationship between the degree of neutropenia induced by peginterferon and infectious AEs. Although several epidemiologic studies have documented lower neutrophil counts among healthy black individuals, constitutional neutropenia in black patients did not appear to increase the risk of serious infections [9-11]. Of the two patients who experienced serious infections, one occurred well after treatment was completed (week 70). Only one serious infection was considered possibly related to treatment. These results confirm those of an earlier study of 119 patients with chronic HCV treated with standard interferon alfa-2a and ribavirin, which showed that the occurrence of neutropenia was not associated with an increased risk of serious infections during therapy [9]. Only 5% (4/78) of black patients prematurely discontinued treatment due to laboratory abnormalities or AEs [7]. Other reasons for premature discontinuation of therapy among blacks were insufficient therapeutic response, failure/refusal to return, withdrawal of consent, and protocol violation. Decreases in haematological parameters observed during treatment were transient and no new safety concerns regarding peginterferon alfa-2a plus ribavirin therapy emerged among black patients infected with HCV genotype 1. Together, these results argue that constitutional neutropenia should not exclude patients from therapy with peginterferon alfa-2a.
The impact of dose modifications of pegylated interferons and ribavirin during treatment on SVR has been a subject of considerable interest [13-15]. Shiffman et al. assessed the efficacy of peginterferon alfa-2a and ribavirin in predominantly white HCV genotype 1 patients who had advanced liver fibrosis and who had failed to clear HCV long-term after previous treatment with either interferon alfa or interferon alfa plus ribavirin [15]. Thirty-six percent of patients had the peginterferon alfa-2a dose reduced and 39% had a reduction in ribavirin dose, in both cases primarily due to peripheral blood cytopenias and anemia. Reductions in ribavirin (but not the peginterferon alfa-2a) dose to 60-80 and ≦60% of the intended dose during the first 20 weeks of treatment were associated with significantly lower SVR compared to patients who maintained a dose ≥80%. Reducing the dose of either medicine after week 24 had no effect on the SVR. McHutchison et al. reported a similar relationship between adherence to peginterferon alfa-2b and ribavirin dose and SVR in HCV genotype 1 patients, but this study did not show a significant relationship between the timing of dose modifications and treatment efficacy [13]. In the current study, peginterferon alfa-2a dose reductions were more common among blacks compared to whites, but the rate of ribavirin dose reduction was similar in blacks and whites. In keeping with studies in predominantly white HCV cohorts, the SVR rate in black patients was related to the adherence to both of peginterferon alfa-2a and ribavirin. Adherence to >60% cumulative exposure to both peginterferon and ribavirin during 48 weeks of treatment was associated with a 30% SVR in black patients. There was no difference in the SVR rates between patients with 61-80 and >80% exposure to both medicines. Ribavirin dose reductions to between 60 and 80% exposure did not appear to decrease the SVR as long as peginterferon exposure was >80% during 48 weeks of therapy. Indeed, three patients who achieved a SVR with ≦60% ribavirin exposure all had >80% peginterferon exposure. Compared to exposure >80%, there was a trend toward a lower SVR when the peginterferon dose was reduced to 61-80% even when ribavirin exposure was >80%. In addition, there was no SVR among patient with ≦60% peginterferon exposure. These results raise the potential that the SVR in black patients might be more sensitive to peginterferon alfa-2a than to ribavirin dose reductions. However, the small number of patients with <80% peginterferon exposure combined with >60% ribavirin exposure limits our ability to determine the relative importance of peginterferon and ribavirin dose reductions to the SVR.
In summary, this trial demonstrated good safety and tolerability of therapy with peginterferon alfa-2a plus ribavirin in blacks with chronic hepatitis C infection. This treatment regimen induced an SVR in 26% of black patients, the highest response to combination therapy yet reported in the black population. Although there is concern that low SVR rates for blacks, 26% in our study [7] and 19% in another study [8] may deter some doctors from offering treatment to black patients, both of trials suggest that black American patients can be treated safely with pegylated interferons plus ribavirin. Our results suggest that the SVR might be improved in black patients by a greater focus on managing the side effects of peginterferon and ribavirin and by increasing patient adherence to treatment. In addition, the lack of association between constitutional and peginterferon-induced neutropenia and infectious AEs suggest a lower ANC threshold for peginterferon dose reductions might be safe. This question merits further study in a prospective clinical trial. Future advances in hepatitis C treatment will occur as new therapies with fewer haematological side effects are developed. New clinical studies are underway to test higher doses of peginterferon alfa-2a and ribavirin, longer courses of therapy, combinations with immunomodulators, and new antiviral drugs to improve virologic response rates.
|
|
| |
| |
|
|
|