| |
Coffee, Cirrhosis, and Transaminase Enzymes
|
| |
| |
Arthur L. Klatsky, MD; Cynthia Morton, MD; Natalia Udaltsova, PhD; Gary D. Friedman, MD
Arch Intern Med. June 12, 2006;166:1190-1195.
ABSTRACT
Background- A minority of persons at risk develop liver cirrhosis, but knowledge of risk modulators is sparse. Several reports suggest that coffee drinking is associated with lower cirrhosis risk.
Methods- We studied 125 580 multiethnic members of a comprehensive prepaid health care plan without known liver disease who supplied baseline data at voluntary health examinations from 1978 to 1985. Subsequently, through 2001, 330 of them were diagnosed with liver cirrhosis. Review of medical records confirmed the diagnosis of cirrhosis and ascertained probable etiology. The association of coffee drinking with cirrhosis was estimated by Cox proportional hazards models with 7 covariates. We also did a cross-sectional analysis of baseline aspartate aminotransferase and alanine aminotransferase levels, studied by logistic regression.
Results.
In the cohort study, relative risks of alcoholic cirrhosis (199 subjects) for coffee drinking (vs none) were less than 1 cup per day, 0.7 (95% confidence interval [CI], 0.4-1.1); 1 to 3 cups, 0.6 (95% CI, 0.4-0.8; P<.001); and 4 or more cups, 0.2 (95% CI, 0.1-0.4; P<.001).
For 131 subjects with nonalcoholic cirrhosis, relative risks were less than 1 cup, 1.2 (95% CI, 0.6-2.2); 1 to 3 cups, 1.3 (95% CI, 0.8-2.1); and 4 or more cups, 0.7 (95% CI, 0.4-1.3). These relative risks for coffee drinking were consistent in subsets. Tea drinking was unrelated to alcoholic or nonalcoholic cirrhosis.
In the cross-sectional analyses, coffee drinking was related to lower prevalence of high aspartate aminotransferase and alanine aminotransferase levels; for example, the odds ratio of 4 or more cups per day (vs none) for a high aspartate aminotransferase level was 0.5 (95% CI, 0.4-0.6; P<.001) and for a high alanine aminotransferase level, 0.6 (95% CI, 0.6-0.7; P<.001), with stronger inverse relations in those who drink large quantities of alcohol.
Conclusion: These data support the hypothesis that there is an ingredient in coffee that protects against cirrhosis, especially alcoholic cirrhosis.
INTRODUCTION
Long-term ingestion of large amounts of alcohol is the most common cause of liver cirrhosis in developed countries,1-6 with the total lifetime ethanol dosage considered to be the key factor.6-9 Women may be more susceptible,10-11 and ethnic differences in risk have been cited.10 The amount of alcohol consumed and an individual's drinking pattern also play a role,2, 12 so it is difficult to definitively establish independence of the demographic traits from these features. Because most long-term heavy alcohol drinkers do not develop cirrhosis,4, 6, 12 there are other predisposing traits. Other possible cofactors include genetic susceptibility, nutritional status, immune-mediated mechanisms, cigarette smoking, diet, and interactions with other hepatotoxins.3-4,6-7,9-10,12-13
In 1992, we reported an inverse relation of coffee drinking to risk of liver cirrhosis in a 10-year cohort follow-up.13 That analysis involved 132 persons who had been hospitalized or who had died from cirrhosis from 1978 to 1988. The inverse coffee-cirrhosis relation was stronger for cirrhosis attributed to alcohol drinking than for non-alcohol-associated disease, but limited statistical power owing to small numbers clouded this differentiation.
An inverse relation of coffee drinking with cirrhosis has been confirmed by studies of nonfatal5, 14-15 and fatal16 disease. A similar inverse relation of coffee drinking to primary hepatocellular carcinoma17-19 warrants mention because of the usual antecedent cirrhosis. There are also reports of lower blood levels of hepatocellular enzymes in coffee drinkers.20-22 Because elevated levels of these enzymes are thought to be a sensitive marker of acute or subacute liver damage, these reports offer further indirect support for possible protection by coffee. The mechanisms for hypothetical protection are speculative, and it is unclear whether the inverse relation is associated more with caffeine or with some other coffee ingredient. The question of whether the inverse relation is specific (or relatively so) for alcoholic liver disease is also unresolved.
We present herein an extension of our cohort study, with a 22-year follow-up and 330 subjects who developed cirrhosis. Rigorous record review was done to verify the presence of chronic liver disease and to assign a probable primary etiology. We also present a cross-sectional analysis of baseline interrelations of coffee, alcohol, and elevated blood levels of liver enzymes.
RESULTS
COFFEE DRINKING AND RISK OF CIRRHOSIS
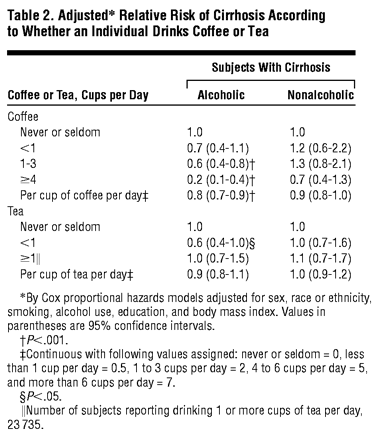
With increasing coffee intake a strong progressive inverse relation to risk of alcoholic cirrhosis was evident (Table 2). There was no significant (P<.05) inverse relation of coffee intake to nonalcoholic cirrhosis. The RR per cup of coffee per day for alcoholic cirrhosis was 0.78 (95% CI, 0.71-0.85); for nonalcoholic cirrhosis it was 0.92 (95% CI, 0.84-1.02). Among subgroups of subjects with nonalcoholic cirrhosis, coffee had a similar, weak, nonstatistically significant inverse relation to risk of either viral hepatitis-associated cirrhosis or to miscellaneous other cirrhosis; the RR per cup per day for both was 0.9 (95% CI, 0.8-1.1). Multivariate regressions with coffee categorized for the 72 subjects with hepatitis showed the following RRs vs non-coffee drinkers: for 1 to 3 cups per day the RR was 1.2 (95% CI, 0.7-2.2; P = .52); for 4 or more cups per day the RR was 0.7 (95% CI, 0.3-1.5; P = .32). Tea intake was unrelated to either alcoholic or nonalcoholic cirrhosis (Table 2).
The inverse relation between coffee and alcoholic cirrhosis showed general consistency in subgroups of sex, ethnicity, age, and BMI category (data not shown). There was no diminution in the lower risk associated with coffee drinking, with increasing intervals between collection of coffee data and cirrhosis diagnosis; RR for more than 4 cups per day vs no coffee at less than 5 years (39 subjects) was 0.2 (95% CI, 0.6-0.5), at 5 to 9 years (59 subjects) it was 0.3 (95% CI, 0.1-0.8), and at more than 10 years (81 subjects) it was 0.2 (95% CI, 0.1-0.5).
The goodness-of-fit test results for alcoholic cirrhosis were satisfactory (2 = 4.62; P = .71).
RISK OF CIRRHOSIS ASSOCIATED WITH COVARIATES
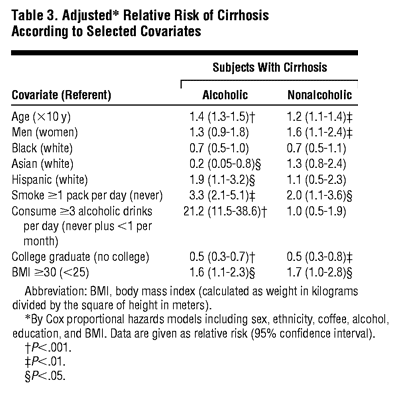
The risk of both alcoholic and nonalcoholic cirrhosis was higher with increasing age, male sex, smoking, and a BMI of 30 kg/m2 or greater, whereas persons with more education had a lower risk of both (Table 3). Asian ethnicity was inversely related and Hispanic ethnicity positively related to alcoholic cirrhosis. Perhaps partially an artifact of classification, intake of large quantities of alcohol was related only to alcoholic cirrhosis.
CORRELATIONS OF HABITS
Coffee drinking was positively correlated with smoking (Pearson correlation coefficient, 0.32) and alcohol drinking (coefficient, 0.18) and was weakly inversely correlated with tea drinking (coefficient, -0.07). The correlation coefficient of smoking with alcohol was 0.30. These correlations were similar for men and for women (data not shown). Coffee drinking was weakly correlated with BMI (+0.06) and unrelated to education (-0.01). Persons who reported drinking 4 or more cups of coffee per day composed 16.3% of all examinees but included substantially disproportionate numbers of heavy smokers and alcohol drinkers. For example, 21.9% (4733 of 21 596) of these heavier coffee drinkers reported smoking at least 1 pack per day (vs 9.2% of all examinees) and 12.2% (2659 of 21 569) reported consuming a least 3 alcoholic drinks per day (vs 8.2% of all examinees).
INVERSE RELATION OF BLOOD LEVELS OF LIVER ENZYMES TO COFFEE DRINKING
As expected, mean blood levels of AST and ALT were progressively higher with heavier alcohol intake, as follows. For lifelong abstainers, the AST level was 20.5 U/L and the ALT level was 15.9 U/L; for ex-drinkers, the AST level was 22.6 U/L and the ALT was level, 18.9 U/L; for those who consumed less than 1 drink per month, the AST level was 20.6 U/L and the ALT level was 16.3 U/L; for those had more than 1 drink per month but less than 1 drink per day, the AST level was 21.0 U/L and the ALT level was 16.7 U/L; for those who had 1 to 2 drinks per day, the AST level was 23.2 U/L and the ALT level was 19.1 U/L; and for those who had 3 or more drinks per day, the AST level was 30.8 U/L and the ALT level was 25.8 U/L.
The mean AST level was 20.1 U/L in men and 24.7 U/L in women; the mean ALT level was 22.4 U/L in men and 14.1 U/L in women. We elected to study the highest 5% of each sex as a definition of high AST and ALT levels. These cutoff points were an AST level of 45 U/L or higher in men or 34 U/L or higher in women, and an ALT level of 52 U/L or higher in men or 31 U/L or higher in women. Comparing heavy alcohol drinkers (3 drinks per day) with abstainers in logistic regression models demonstrated statistically significant (P<.001) increased prevalence of high values: an odds ratio for the AST level of 3.9 U/L (95% CI, 2.9-4.8) and for the ALT level of 2.2 U/L (95% CI, 2.0-2.6). In logistic models stratified by alcohol intake we found a substantial inverse relation of coffee drinking to prevalence of high AST and ALT levels within the alcohol intake strata (Table 4). This inverse relation of coffee to elevated enzyme levels was strongest in heavier alcohol drinkers (Table 4).
COMMENT
Our data showed a robust inverse relation of coffee drinking to risk of alcoholic cirrhosis, independent of several potential confounders. In contrast, there was no statistically significant relation of coffee drinking to risk of nonalcoholic cirrhosis. The positive correlations of coffee drinking with traits related to increased cirrhosis risk, such as alcohol intake, smoking, and BMI, indicate that residual confounding for these traits would more likely result in a positiveĀ\not an inverse-coffee-cirrhosis relation. One analysis of potential confounders of health studies of coffee consumption26 led to the conclusion that only smoking and sex were of importance. Another report27 showed a positive correlation among long-term alcoholic subjects between alcohol and caffeine intake, similar to those of persons with and without a family history of alcoholism; those authors inferred the likely presence of a behavioral rather than a genetic interaction between these behaviors. These considerations, in concert with the strength of the inverse coffee-alcoholic cirrhosis association, make confounding by associated habits an unlikely explanation.
Our cross-sectional data about coffee drinking and high blood levels of liver enzymes are generally comparable with other reports20-22 and inferentially support a possible protective effect of coffee against hepatocyte damage. Lower prevalence of elevated enzyme levels among coffee drinkers was not limited to heavy alcohol drinkers in our study population, but the apparent coffee effect was stronger among them. It is probable that some heavy alcohol drinkers underreport their intake. If only persons with heavy alcohol intake had elevated liver enzyme levels and hepatocyte protection by coffee, such underreporting could produce an apparent, but spurious, inverse relation of coffee to high AST and ALT levels among lighter alcohol drinkers or abstainers. However, it seems unlikely that underreporting of alcohol intake is the entire explanation, and the enzyme level relations suggest less specificity of coffee's apparent protection against acute or subacute alcohol-induced damage. It remains possible that coffee is more specifically protective against severe chronic liver disease (ie, cirrhosis) when alcohol is the noxious agent.
In other reports,5, 16-17 specificity of the inverse coffee-cirrhosis relation for alcoholic liver disease has not been consistently found. Lower prevalence of an elevated ALT level in coffee drinkers in one report22 was similar for various high-risk groups for liver disease, such as heavy alcohol drinkers, those with chronic viral hepatitis, or obese persons. The inverse relation of coffee to hepatocellular carcinoma17-19 argues indirectly against specificity for alcohol-induced disease, especially because 2 of these reports18-19 are from Japan, where chronic viral disease plays a preponderant role in this malignancy.28
The absent relation of tea drinking to cirrhosis might mean that the relation is less likely due to caffeine than to some other coffee ingredient. However, substantial tea drinking was relatively uncommon in this population, and tea typically has less caffeine per cup than coffee.29 A recent report30 presented data showing a similarly decreased relative risk of liver disease for caffeinated coffee as for tea, with an RR of more than 2 cups per day vs less than 1 cup per day of coffee of 0.5 (95% CI, 0.3-1.0) and of tea of 0.6 (95% CI, 0.1-2.3). As those authors stated,30 the tea data lack statistical significance, but the issue of a possible relation of tea to liver disease remains open. Information about coffee and tea type was available for about 10% of our study population. Although the number of subjects with cirrhosis among that 10% was insufficient for analysis, we do know that heavier coffee drinkers in this population were less likely to drink decaffeinated coffee.29 Previous reports5, 16, 22 are disparate with respect to whether the apparently protective coffee ingredient is caffeine; in our opinion this issue is quite unresolved.
Approximately 80% of Americans consume coffee, averaging 3.2 cups per day.31 On average, 80% of all caffeine is taken as coffee,29 so that coffee drinking is sometimes considered a marker for caffeine intake. Because of the evident stimulatory effects of caffeine, this prominent coffee component has been much studied, but coffee is a complex substance with many potentially biologically active ingredients.32 The fact that coffee is also frequently taken with added cream, milk, sugar, or other substances adds more possibilities for health effects. Speculations22 about hepatic protection include caffeine effects via adenosine receptor antagonists or antioxidant actions and potential adverse effects of cafestol (a fat-soluble noncaffeine component of boiled coffee) via induction of glutathione, a cellular protective factor.
The positive association of smoking to risk of alcoholic cirrhosis is clearer here than in our earlier report,13 with a positive, albeit weaker, relation to nonalcoholic cirrhosis. Because smoking and alcohol drinking are related habits, it is difficult to completely rule out residual confounding by alcohol amount or drinking pattern. Coffee drinking and smoking are also strongly correlated, and smoking may prolong the persistence of caffeine in the body.33 Thus, any residual confounding related to coffee drinking would tend to produce an inverse smoking-cirrhosis relation, not the positive one we obtained.
Ascertainment of habits only at baseline time is a limitation of our study, but the probable effect of cessation or reduction of coffee drinking after baseline but before diagnosis would be to weaken, not to strengthen or produce, the inverse coffee-cirrhosis association we found. A second limitation is lack of information about changes in coffee drinking habits before baseline evaluation. Hypothetically, some persons with symptoms of undiagnosed liver disease might have reduced coffee intake or quit drinking coffee, thus spuriously increasing the risk of the non-coffee-drinking referent group. The probable result of such a phenomenon would be an apparent strong inverse coffee-cirrhosis relation only in the first years after baseline time; persistence of the inverse relation among persons with at least 10 years to diagnosis argues against this. A third limitation is the use of hospitalization and death as end points, leaving unexplored the relation of coffee drinking to milder liver disease. A fourth limitation is incomplete follow-up of the cohort, but it seems unlikely that this is systematically related to coffee drinking. Yet another limitation is the inability to account for changes in alcohol drinking or inaccurate reporting of alcohol drinking. We have evidence that alcohol drinking habits are relatively stable in this study population.34 The fact that some subjects with alcoholic cirrhosis reported lifelong abstinence and light drinking (Table 1) suggests possible underreporting. However, assuming correct diagnosis, increase or onset of drinking after baseline in some subjects could be the explanation.
The observational nature of the data and the absence of an established mechanism limit a causal interpretation. There are also no clear therapeutic implications; even if coffee is protective, the primary approach to reduction of alcoholic cirrhosis is avoidance or cessation of heavy alcohol drinking. Assuming causality, the data do suggest that coffee intake may partly explain the variability of cirrhosis risk in alcohol consumers. Basic research about hepatic coffee-ethanol interactions is warranted, but we should keep in mind that coffee might represent only one of a number of potential cirrhosis risk modulators.
METHODS
SUBJECTS AND DATA FOR COHORT STUDY
The study protocols were approved by the institutional review board of the Kaiser Permanente Medical Care Program, Oakland, Calif. Baseline data were from health appraisal examinations23 voluntarily taken by members of a Northern California prepaid comprehensive health care program. These members are believed to reflect the full socioeconomic spectrum of the general population except for the extremes of income.24 From 1978 to1985 a research alcohol questionnaire was completed by 125 580 adults who responded negatively to queries about presence of liver disease. These individuals comprised over 80% of all examinees; the rest consisted largely of individuals taking the examination in the absence of a special research clerk or those who were not fluent in English. The basic examination included health measurements and queries about sociodemographic status, habits, and medical history.23 The research questionnaire included self-classified ethnicity and additional data about alcohol, coffee, and tea use in the past year (alcohol use defined as the usual number of drinks per day; coffee and tea consumption, as the usual number of cups per day).
ASCERTAINMENT OF DIAGNOSES OF CIRRHOSIS
We attempted to identify all persons among the 128 934 examinees who had a first hospital admission for cirrhosis or who died of cirrhosis after the 1978-1985 baseline examination through December 31, 2002. Excluded were persons with a primary or secondary International Classification of Diseases, Ninth Revision (ICD-9),25 code 571 diagnosis (chronic liver disease and cirrhosis) prior to the baseline examination. A computer search was performed for code 571 primary hospitalization discharge diagnoses in Northern California Kaiser Permanente databases and in California death indices. We also screened persons with other diagnoses considered likely to represent underlying liver cirrhosis; these included code 155.1 (malignant neoplasm of liver, primary), 456.0-456.2 (esophageal varices), 572.2 (hepatic coma), 572.3 (portal hypertension), and 572.4 (hepatorenal syndrome). The diagnosis of cirrhosis was considered confirmed if (1) there was histological confirmation by autopsy or liver biopsy, (2) there were at least 2 hospital admissions more than 12 months apart for the condition or 1 admission plus a death certificate diagnosis, (3) the diagnosis was made by a gastroenterologist, or (4) there was a compelling clinical picture. A compelling clinical picture required, in the absence of other explanations, the presence of at least 4 of the following: spider nevi, scleral icterus, palmar erythema, ascites, flapping tremor, hepatosplenomegaly, a platelet count of less than 140 000/É L, ultrasonographic findings of a portal vein diameter larger than 12 mm, a serum albumin level below 3.0 g/dL (35 g/L), and an international normalized ratio prothrombin time greater than 1.5
Probable cirrhosis etiology was determined by physician investigators with the aid of computer-stored information and complete paper inpatient and outpatient medical records. Consideration was given to data about alcohol-drinking history, alcohol-related diagnoses, and clinical data at the time of diagnosis. Uncertain cases were resolved by consensus involving a gastroenterologist. The opinion of a gastroenterologist in the medical record was given considerable weight but was sometimes changed. When both chronic viral hepatitis and a history of substantial alcohol drinking were present, the probable etiology was assigned as nonalcoholic. Of 430 persons initially screened, 100 were excluded, leaving in the cohort study 330 subjects who developed cirrhosis. The largest groups excluded were (1) former health plan members with a diagnosis of cirrhosis on their death certificates but no confirmatory evidence in plan records (n = 39), persons with transient alcoholic hepatitis (n = 38), cases with other primary malignancy, misclassified as hepatoma (n = 9), those with missing medical records (n = 6), and those with other misdiagnoses (n = 7). Of the subjects with nonalcoholic cirrhosis, 72 were judged to have chronic viral disease, 11 had biliary cirrhosis, 4 each had hemochromatosis or autoimmune disease, and 40 had cryptogenic cirrhosis. Table 1 shows additional selected traits of the total study population and of the subjects with cirrhosis.
BASELINE LIVER ENZYMES
At baseline examinations from January 1978 through October 1979, a total of 37 620 persons had aspartate aminotransferase (AST) level determinations. From January 1978 through August 1981, 69 904 persons had alanine aminotransferase (ALT) level determinations. Except for the examination date, there were no other selection factors.
ANALYTIC METHODS FOR COHORT STUDY
Each subject was followed up until December 31, 2001, or until death, other health plan termination, or first hospitalization for cirrhosis, whichever occurred first. This yielded a total of 1 820 201 person-years of observation (mean follow-up time, 14.1 years). For age-adjusted and multivariate analyses, we used Cox proportional hazards models determined by the PHREG procedure of the SAS Users Guide (version 8; SAS Institute, Inc, Cary, NC). Most multivariate models included age (x 10 years), sex, race (referent, white; black, Asian, Hispanic, or other), education (referent, no college; some college or college graduate), body mass index (BMI), calculated as weight in kilograms divided by the square of height in meters (referent, <25; 25-29, or >30), alcohol (referent, never drank or <1 drink per month; <1 per day, 1-2 per day, or >3 per day), and cigarette smoking (referent, never smoked; ex-smoker, <1 pack per day, or >1 pack per day). Each covariate was included because of the apparent significant (P<.05) effect of alcoholic cirrhosis in stepwise regression analyses. The model fit was assessed using the Hosmer-Lemeshow goodness-of-fit test. The analyses yielded estimates of relative risk (RR), 95% confidence intervals (CIs), and P values.
For cross-sectional liver enzyme analysis, elevated levels of AST and ALT, defined as the highest 5% for each sex, were studied by logistic regression with categories of age, race, smoking, and alcohol drinking as covariates; these models were determined for all persons in each sex and at specific reported drinking levels.
In addition to direct cross-classification, correlations of cigarette smoking and alcohol, coffee, and tea drinking were studied as Pearson correlation coefficients.
|
|
| |
| |
|
|
|