| |
Hepatic Steatosis Is Associated with Fibrosis, Nucleoside Analogue Use, and Hepatitis C Virus Genotype 3 Infection in HIV-Seropositive Patients:
Steatosis was present in 69% of patients
|
| |
| |
Clinical Infectious Diseases August 1, 2006;43:365-372
Barbara H. McGovern,1,4 Jeremy S. Ditelberg,4,5 Lynn E. Taylor,7,8 Rajesh T. Gandhi,2,6 Katerina A. Christopoulos,2,6 Stacey Chapman,7 Beth Schwartzapfel,7 Emily Rindler,4 Anne-Marie Fiorino,4 M. Tauheed Zaman,1,2 Paul E. Sax,3,6 Fiona Graeme-Cook,2,6 and Patricia L. Hibberd4,5
1Lemuel Shattuck Hospital, Jamaica Plain, and 2Massachusetts General Hospital, 3Brigham & Women's Hospital, 4Tufts-New England Medical Center, 5Tufts University School of Medicine, and 6Harvard School of Medicine, Boston, Massachusetts; and 7The Miriam Hospital and 8Brown Medical School, Providence, Rhode Island
".....In this large, racially diverse cohort of HIV-HCV-coinfected patients, most of whom acquired HCV infection through injection drug use, we demonstrate that steatosis is associated with nuceloside analogue use and HCV genotype 3 infection. In addition, we confirm and extend the findings of Sulkowski et al. [28] that steatosis is associated with liver fibrosis. These data are of vital clinical importance, because fibrosis progression occurs faster in HIV-HCV-coinfected patients than in patients with HCV infection alone. Steatosis was more prevalent in our multiracial cohort than in Sulkowski and colleagues' mainly African American cohort (68% vs. 40% prevalence)..... administration of antiretroviral agents with little or no mitochondrial toxicity (e.g., tenofovir, lamivudine, emtricitabine, or abacavir) may be preferred over other NRTIs whenever possible...."
ABSTRACT
Background. We conducted a study to determine the prevalence and factors associated with hepatic steatosis in human immunodeficiency virus (HIV)-seropositive patients with hepatitis C and to investigate whether steatosis is associated with liver fibrosis.
Methods. Retrospective chart reviews were conducted in 4 hospitals that serve community-based and incarcerated HIV-infected patients who had undergone a liver biopsy for evaluation of hepatitis C virus (HCV) infection during the period of 2000-2003. Demographic characteristics and medication and laboratory data were collected from the time of the biopsy. A pathologist blinded to all clinical data evaluated the specimens. The primary outcome was presence or absence of steatosis.
Results.
Of 260 HIV-HCV-coinfected patients, 183 met inclusion criteria and had a biopsy specimen adequate for review.
Steatosis was present in 69% of patients (graded as minimal in 31%, mild in 27%, moderate in 18%, and severe in 1%).
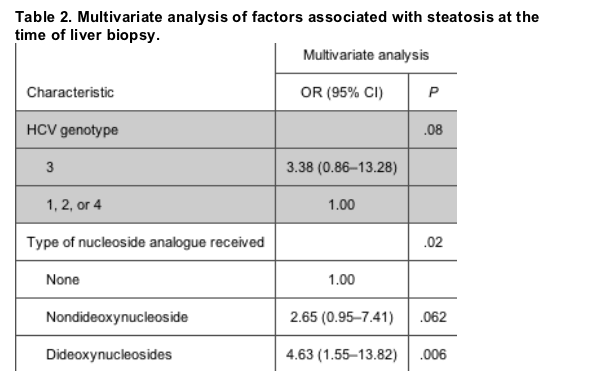
Factors associated with steatosis included use of dideoxynucleoside analogues, such as didanosine and stavudine (odds ratio [OR], 4.63; 95% confidence interval [CI], 1.55-13.82).
There was a trend toward presence of steatosis and use of other nucleoside analogues or infection with HCV genotype 3 (OR, 2.65 [95% CI, 0.95-7.41] and 3.38 [95% CI, 0.86-13.28], respectively).
The presence of steatosis was associated with fibrosis (OR, 1.37; 95% CI, 1.03-1.81).
Conclusions. In this multiracial population of HIV-HCV-coinfected patients, steatosis was prevalent and was associated with severity of liver fibrosis. Use of nucleoside analogues (particularly didanosine and stavudine) and HCV genotype 3 infection were associated with hepatic steatosis. The development of steatosis is multifactorial in nature and may play a contributory role in the progression of liver disease in HIV-infected patients.
Factors associated with hepatic steatosis.
In univariate analysis, presence of steatosis was associated with greater weight and a nondetectable HIV RNA level (table 1). There was a trend towards presence of steatosis in patients with higher BMI for the 82 subjects for whom both height and weight were available (P = .07). The relationship between steatosis and HCV genotype 3 infection was of borderline significance (univariate OR, 2.48; 95% CI, 0.88-6.94; P = .08), as it was for glucose and triglyceride levels.
Use of any antiretroviral agent, any nucleoside analogue, and any type of nucleoside analogue were associated with steatosis. When types of nucleoside analogues were stratified into non-dideoxynucleoside analogues versus dideoxynucleoside analogues, the risk of steatosis increased with the dideoxynucleosides. The strength of this relationship increased with the use of 2 dideoxynucleosides (OR for didanosine and stavudine, 3.38; 95% CI, 0.67-17.07) than with the use of either medication alone (univariate OR, 2.50; 95% CI 1.09-5.76). Although only 21 patients were taking triple-nucleoside analogue therapy, 19 had evidence of hepatic steatosis (univariate OR, 7.13; 95% CI, 1.51-33.57).
On multivariate analysis, nucleoside analogue use was associated with hepatic steatosis (table 2). When comparing use of non-dideoxynucleoside analogues with no nucleoside analogue use, the multivariate OR was 2.65 (95% CI, 0.95-7.41; P = .062); when comparing dideoxynucleoside analogue use with no nucleoside analogue use, the OR was 4.63 (95% CI, 1.55-13.8; P = .006). There was also a trend for an association with HCV genotype 3 infection (P = .08). The model fit the data well, with a Hosmer Lemeshow goodness of fit test P value of .97 and an area under the receiver operating characteristic curve of 0.64 (95% CI, 0.55-0.73; P = .005).
Relationship between steatosis and fibrosis. Presence and absence of steatosis was compared with extent of fibrosis and necroinflammation by Knodell stage and grade. Steatosis was associated with stage of fibrosis (univariate OR, 1.37; 95% CI, 1.03-1.81; P = .029) but not with grade of necroinflammation (univariate OR, 1.09; 95% CI, 0.95-1.25; P = .23). Fibrosis progression rates could be calculated for 56% of the sample (because of missing data about the duration of HCV infection on 80 charts) and was found to be associated with steatosis.
INTRODUCTION
In patients infected with hepatitis C virus (HCV) alone, hepatic steatosis (fatty liver) results in accelerated progression of liver disease [1-4]. HCV genotype 3 [1, 4] and host factors, including increased body mass index (BMI), visceral adiposity, hyperlipidemia, and peripheral insulin resistance [1, 3, 5], are associated with hepatic steatosis. Because these same morphologic and metabolic abnormalities coexist in HIV lipodystrophy syndrome, HIV-HCV-coinfected patients may be at risk of developing steatosis [6, 7]. HAART may be another potential contributor to hepatic steatosis [8, 9]. Nucleoside analogues with high affinity for mtDNA polymerase- (e.g., didanosine and stavudine) cause abnormal deposition of triglycerides in hepatocytes as a result of mitochondrial toxicity [10-13]. The histologic finding of microvesicular steatosis suggests the presence of oxidative stress and energy crisis, which lead to liver injury [10, 14-17]. Mitochondrial toxicity can rarely manifest as life-threatening hepatomegaly with lactic acidosis; however, the full spectrum of fatty liver disease associated with antiretroviral drug administration has not been established [8, 18-20].
The relationship between steatosis and progression of liver disease in HIV-HCV-coinfected patients is unclear [21, 22]. Fibrosis progression rates are faster in HIV-HCV-coinfected patients, compared with patients infected with HCV alone [23]; to date, this difference has been mostly attributed to HIV-related immunosuppression [23-27]. Sulkowski et al. [28] recently found a low prevalence of steatosis in their cohort of predominantly African American patients with HCV genotype 1 infection; however, steatosis was associated with white race, and there was an increased rate of fibrosis among patients who had fatty liver. The use of stavudine and protease inhibitors was also associated with steatosis [21]. Monto et al. [29] were unable to confirm this relationship in their study of 92 patients, and the prevalence of steatosis in their study was low. In both studies, the observed prevalence of steatosis may be partially explained by racial composition, because African American patients with HCV monoinfection are at lower risk of steatosis than are white patients [30].
The objective of this study was to evaluate the factors associated with hepatic steatosis and its relationship to fibrosis in our multiracial, longitudinal cohort of 260 HIV-HCV-coinfected patients. Subjects were drawn from clinics with high rates of injection drug use in Massachusetts and Rhode Island. Our specific aims were (1) to determine the prevalence of hepatic steatosis, (2) to identify factors associated with steatosis, and (3) to assess whether steatosis is associated with fibrosis.
RESULTS
Study population. A total of 260 HIV-infected patients with HCV coinfection who had undergone a liver biopsy were evaluated for inclusion. Sixty-seven patients were omitted from further consideration owing to the exclusion criteria (figure 1); 35 of these patients were eligible except for availability of the biopsy specimen and had demographic characteristics similar to those of the eligible subjects. Of the 193 eligible patients, 10 were not analyzed because the biopsy specimen was inadequate for analysis. The demographic and laboratory data for subjects with and subjects without steatosis are shown in table 1. The median age was 43 years; 79% of the subjects were male; and 50% of the subjects were white, 27% were Hispanic, and 24% were African American. Eighty-three percent of the subjects had a history of injection drug use; for injection drug users, the median duration of HCV infection was 23 years. HCV genotype distribution was as follows: genotype 1, 60%; genotype 2, 8%; genotype 3, 16%; and genotype 4, 4%; the genotype was not available for 12%. At the time of the liver biopsy, 55 patients (30%) were not taking HAART.
Presence and severity of steatosis. At 10X magnification, steatosis was present in 69% of the biopsy specimens and was graded as minimal in 31%, mild in 27%, moderate in 18%, and severe in 1%. The interobserver agreement ( statistic) between the two pathologists for the 24 specimens read by both was 0.47 (highly significant agreement P = .02). The pattern of steatosis was also evaluated at 40X magnification and graded as highly significant between the readers (Somer's 0.78, P < .001). A pure macrovesicular pattern was found in 4% and pure microvesicular in 19%. The vast majority (50%) of specimens had a mixed pattern.
DISCUSSION
In this large, racially diverse cohort of HIV-HCV-coinfected patients, most of whom acquired HCV infection through injection drug use, we demonstrate that steatosis is associated with nuceloside analogue use and HCV genotype 3 infection. In addition, we confirm and extend the findings of Sulkowski et al. [28] that steatosis is associated with liver fibrosis. These data are of vital clinical importance, because fibrosis progression occurs faster in HIV-HCV-coinfected patients than in patients with HCV infection alone. Steatosis was more prevalent in our multiracial cohort than in Sulkowski and colleagues' mainly African American cohort (68% vs. 40% prevalence).
Nucleoside reverse-transcriptase inhibitors (NRTIs) as a class were associated with the presence of steatosis, which is consistent with in vitro and in vivo data for zidovudine, stavudine, and didanosine [11, 20, 34, 35]. This clinical observation is supported by in vitro and in vivo data that suggest that didanosine and stavudine have significant mitochondrial toxicity that exceeds that of other drugs [10, 36-38].
Our finding that a nondetectable HIV RNA level was associated with steatosis is probably reflective of antiretroviral medication use, as noted by others [29]. We were not able to confirm any association between PI use and hepatic steatosis [28]. Finally, we observed a trend toward an association between hepatic steatosis and host and metabolic factors, such as hypertriglyceridemia, increased glucose concentration, and increased BMI, as noted in patients infected with HCV alone [39].
Microvesicular steatosis was highly prevalent in our patient population. Because this histologic pattern implies the presence of mitochondrial dysfunction, these data lead to mounting concerns regarding long-term administration of nucleoside analogues with a propensity for mitochondrial toxicity [40]. Much has been written about the depletion of mtDNA in PBMCs and adipocytes secondary to mitochondrial toxicity of HAART [38]. However, this is the first large study of HIV-HCV-coinfected patients to show that mitochondrial dysfunction, as evidenced by microvesicular steatosis, is present in the target organ of concern.
Because liver disease is a significant comorbidity in HIV-HCV-coinfected patients, it is vitally important to understand the implications of hepatic steatosis and its impact on liver disease progression [41]. Steatosis of any etiology can be associated with development of necroinflammatory change and fibrosis [2, 42, 43]. It supplies "fuel for the fire" of lipid peroxidation, which leads to stellate cell activation, collagen synthesis, and fibrosis [44, 45]. We propose that HIV-infected patients may be at increased risk for steatosis and subsequent liver injury through 2 different pathways: one mediated through HCV infection, and another via the use of nucleoside analogues [42, 46, 47].
The potential contributors to this process fall into 3 main categories. First, there is evidence that HCV itself leads to steatosis via a direct cytopathic effect on hepatocytes or a modification of lipoprotein assembly and secretion [48-50]. In vitro and animal studies demonstrate that HCV core and nonstructural proteins can induce steatosis via mitochondrial injury, oxidative stress, and production of reactive oxygen species [46, 48, 51, 52]. Clinical studies confirm the association of HCV genotype 3 and steatosis and its regression with successful treatment and viral eradication [4, 53]. Second, host factors, such as elevated BMI, visceral obesity, insulin resistance, and hyperlipidemia, may contribute to hepatic steatosis in HIV-infected patients [6]. These metabolic abnormalities are recognized clinically as HIV lipodystrophy syndrome. Finally, the administration of nucleoside analogues-particularly didanosine and stavudine-may contribute to the development of microvesicular steatosis, lipid peroxidation, and, with prolonged duration, steatohepatitis [40].
Steatosis, as the "first hit," primes hepatocytes for the "second hit": oxidative stress [54]. Steatosis is the substrate for lipid peroxidation, which results in the basal formation of potentially harmful reactive oxygen species that lead to liver injury [17]. Oxidative stress occurs when there is an imbalance between increased production of reactive oxygen species and depleted antioxidant defenses, such as glutathione, that prevent damage from these oxygen radicals [55]. HIV-infected patients are at increased risk of oxidative stress as a result of depressed stores of protective antioxidant scavengers [11, 56, 57]. When antioxidants are depleted, excess reactive oxygen species can damage mtDNA and oxidize fat, causing a perpetual cascade of increased lipid peroxidation, oxidative stress, and hepatocellular injury [11]. Long-term nucleoside analogue use depletes baseline levels of mtDNA, thereby increasing patient susceptibility to liver injury [58]. Other potential contributors to oxidative stress include alcohol use and chronic inflammatory cells related to HCV [59]. We propose a model to explain faster fibrosis progression rates seen in patients with HIV and HCV coinfection (figure 2).
The limitations of our study included the following: (1) a trend for significance of BMI and steatosis was found, but this parameter could only be calculated for 82 patients at 1 site (Lemuel Shattuck Hospital); (2) histories of alcohol use obtained from a chart review may underestimate the prevalence of heavy alcohol intake; (3) glucose measurements were not always designated as fasting or random; and (4) electron microscopy was not performed to look for morphologic aberrations of mitochondrial structures.
Our data linking an association between steatosis and dideoxynucleoside use are also limited by the cross-sectional design of our study. Any association between steatosis and dideoxynucleoside use will need to be confirmed by longitudinal prospective studies. The impact of prior or cumulative NRTI exposure could not be evaluated in our study and was likely important. Future prospective studies of the link between lipodystrophy syndrome and hepatic steatosis should include measurements of insulin resistance, fasting glucose level, and triglyceride level, as well as waist-hip circumference and BMI. Whether prior chronic exposure to dideoxynucleosides has durable effects on the severity of hepatic steatosis is unknown.
Although our data highlight potential concerns regarding nucleoside analogue therapy on hepatocytes, liver fibrosis progression rates are slower in patients in the HAART era [60, 61]. These outcomes have been attributed solely to immune recovery. However, it should also be noted that successful control of HIV infection contributes to suppression of TNF-, an important mediator of hepatic oxidative stress and the cascade of events that lead to lipid peroxidation and subsequent fibrosis [62]. Suppression of HIV replication by HAART may contribute to slower fibrosis progression via TNF- suppression, as well as T cell restoration. Whether the use of alternative nucleoside analogues and avoidance of the "D-drugs" will increase the benefits of HIV control without the additional harm of mitochondrial toxicity remains to be seen [63].
Our data have practical implications for the management of HIV-HCV-coinfected patients. Treatment of the patient with genotype 3 infection should be encouraged, because efficacy is high in this group and viral eradication may be associated with regression of steatosis [64]. Secondly, although some have proposed noninvasive markers for the assessment of fibrosis, we still prefer liver biopsy as the gold standard, because this is the only way to adequately assess the degree of steatosis and its histologic pattern. Finally, administration of antiretroviral agents with little or no mitochondrial toxicity (e.g., tenofovir, lamivudine, emtricitabine, or abacavir) may be preferred over other NRTIs whenever possible [11, 63, 65].
METHODS
Demographics of study population. Study subjects were HIV-infected patients who underwent liver biopsy during the period of January 2000 through December 2003 for evaluation of HCV infection at 4 outpatient clinic sites, including: Lemuel Shattuck Hospital (Jamaica Plain, MA), Massachusetts General Hospital (Boston, MA), Miriam Hospital (Providence, RI), and Brigham and Women's Hospital (Boston, MA). Medical records of individuals aged >18 years were screened for the following inclusion criteria: the patient had HIV and HCV infection, and liver biopsy slides were available for review by the study pathologist. HCV infection was confirmed by both ELISA and quantitative HCV RNA (Amplicor, version 2; Roche); HIV infection was confirmed by either serologic testing or presence of HIV RNA. Exclusion criteria were as follows: (1) chronic hepatitis B virus infection, other cause of liver disease, or abnormal serum iron, ferritin, a-1 antitrypsin, ceruloplasmin, or anti-nuclear antibody levels; (2) history of taking steatosis-inducing drugs, or (3) findings consistent with cirrhosis (spider angiomata or splenomegaly). Research assistants were trained to abstract the data from medical records (including demographic characteristics, laboratory findings, and data on medications) onto study-specific case report forms. HAART was defined as a 3-drug regimen that included 1 or 2 nucleoside analogues plus a protease inhibitor (PI) and/or a nonnucleoside reverse-transcriptase inhibitor (NNRTI). Medical history data included weight, height (when available), alcohol use, and risk factors for HCV infection. All reviewed records were included in the screening log.
All study sites reviewed this protocol and issues of patient confidentiality. This protocol conforms to the 1975 Helsinki guidelines for the conduct of human research and was approved by the investigational review boards of all participating hospitals, including Lemuel Shattuck Hospital, which includes a prisoner advocate.
Laboratory data. Data abstracted from the chart were those closest in date and before the liver biopsy (preferably, up to a maximum of 6 months before the procedure or 3 months after). Tests included complete blood cell counts; determination of aminotransferase, bilirubin, alkaline phosphatase, prothrombin, and HIV RNA levels and CD4+ T cell count; and determination of HCV antibody, quantitative HCV RNA level, and HCV genotype. For glucose, cholesterol, and triglyceride levels, the charts usually did not specify whether fasting or random samples were obtained. A nondetectable HIV RNA level was defined as the lower limit of detection for the individual assay, although this cutoff varied from <50 to <400 copies/mL.
Liver histopathologic examination. Biopsy specimens that were stained with hematoxylin and eosin, Masson's trichrome, and Prussian blue iron stains were included for review using a standardized protocol. Specimens that did not include 5 portal tracts were considered to be unacceptable. Steatosis was assessed as absent, minimal, mild, moderate, or severe at light magnifications of 10X and 40X, in accordance with the protocol of Brunt et al. [31]. In accordance with the protocol of Knodell et al. [32], the fibrosis stage was assigned on the basis of a scale from 0 to 4 (for no fibrosis to cirrhosis), and necroinflammatory changes were graded on a scale from 0 to 18 (for no changes to severe changes). The pattern of steatosis was assessed under 40X magnification and described as "microvesicular," "macrovesicular," or "mixed" [31].
Biopsy specimens were assessed by 1 study pathologist (J.S.D.). A second pathologist (F.G.C.) assessed a random sample of 24 biopsy specimens (10% of the study sample) for internal consistency. The pathologists recorded data on study-specific case report forms. Both were blinded to clinical data.
Statistical analysis. The primary outcome for the study was presence (assessed as minimal, mild, moderate, or severe) or absence of steatosis on a biopsy specimen (magnification, X10). To evaluate study power, because the sample size for this study was fixed at 183 patients (i.e., all eligible patients seen at the study clinics), we evaluated our ability to detect differences in antiretroviral use in subjects with and without steatosis. We assumed that >90% of subjects with steatosis would be receiving at least 1 antiretroviral agent at the time that the liver biopsy was performed. Our hypothesis was that patients without steatosis would be less likely to be taking dideoxynucleoside analogue agents. With the available sample size of 183 patients (with about 90 anticipated to have steatosis and about 90 to not have steatosis), we calculated that we would have >80% power to detect a difference in antiretroviral drug use in the 2 groups of at least 20% (i.e., were anticipated rates of 90% in the steatosis group and 70% in the nonsteatosis group). We anticipated that a sample size of 183 subjects was also sufficient to conduct multivariate analysis with up to 9 variables.
Factors associated with the presence versus the absence of steatosis were compared in a univariate analysis using ORs, 95% CIs, and 2-sided P values. Factors analyzed included the subject's sex, age, BMI, and race/ethnicity; heavy alcohol use; diabetes; HIV RNA level, CD4 cell count, and glucose, cholesterol, and triglyceride levels; duration of HCV infection; HCV RNA level; HCV genotype (genotype 3 vs. all others); and use of any antiretroviral agent, HAART, any PI, any NNRTI, or any nucleoside analogue. A multivariate logistic-regression model (stepwise algorithm) was used to evaluate which factors were associated with presence of steatosis. Prespecified covariates (antiretroviral drug use, HAART, and infection with HCV genotype 3) were evaluated for inclusion in the multivariate model, as were variables with a P value of <.05 on univariate analysis. Variables retained in the final models had a P value of <10. Statistical analyses were performed using SPSS software, version 12.0 (SPSS). The relationship between steatosis and fibrosis was evaluated using a 2-sided x2 test. The rate of fibrosis progression was calculated as the Knodell stage divided by the duration of HCV infection in years [33] and was compared in the 2 groups using a Wilcoxon rank sum test, because the data were not normally distributed.
|
|
| |
| |
|
|
|