| |
Cost effectiveness of interferon or peginterferon with ribavirin for histologically mild chronic hepatitis C
|
| |
| |
Gut Sept 2006;55:1332-1338
R Grieve1, J Roberts1, M Wright2, M Sweeting3, D DeAngelis4, W Rosenberg5, M Bassendine6, J Main2 and H Thomas2
1 Health Services Research Unit, London School of Hygiene and Tropical Medicine, London, UK
2 Department of Medicine, Imperial College, London, UK
3 MRC Biostatistics Unit, Cambridge, UK
4 Statistics, Modelling and Bioinformatics Department, Health Protection Agency, London, UK, and MRC Biostatistics Unit, Cambridge, UK
5 Division of Infection Inflammation and Repair, University of Southampton, Southampton, UK
6 School of Clinical Medical Sciences, The Medical School, Newcastle upon Tyne, UK
ABSTRACT
Background: For patients with mild chronic hepatitis C the cost effectiveness of antiviral therapy is unknown.
Aims: To assess whether antiviral therapy (either interferon or peginterferon combined with ribavirin) is cost effective at a mild stage compared with waiting and only treating those cases who progress to moderate disease.
Patients: Cases with mild chronic hepatitis C.
Methods: A cost effectiveness model which estimates long term costs and outcomes for patients with mild chronic hepatitis C. The model uses effectiveness and cost data from the UK mild hepatitis C randomised controlled trial, combined with estimates of disease progression and cost from observational studies.
Results: Antiviral treatment at a mild rather than a moderate stage improved outcomes measured by quality adjusted life years (QALYS) gained. The mean cost per QALY gained from antiviral treatment with interferon a-2b and ribavirin, compared with no treatment at a mild stage, was £ 4535 ($7108) for patients with genotype non-1 and £ 25 188 ($39 480) for patients with genotype 1. Providing peginterferon -2b and ribavirin at a mild rather than a moderate stage was also associated with a gain in QALYS; the costs per QALY gained were £ 7821 ($12 259) for patients with genotype non-1 and £ 28 409 ($44 528) for patients with genotype 1.
Conclusions: For patients with chronic hepatitis C, it is generally more cost effective to provide antiviral treatment at a mild rather than a moderate disease stage. For older patients (aged 65 years or over) with genotype 1, antiviral treatment at a mild stage is not cost effective.
Antiviral therapy, either interferon or peginterferon in combination with ribavirin, has been shown to be effective,4-7 and cost effective for patients with chronic hepatitis C,8,9,10 and has been recommended for patients with moderate disease or cirrhosis.11-14 However, there is no clear guidance on whether patients with histologically mild chronic hepatitis C should be treated.11-14 The efficacy of antiviral therapy is similar for patients with mild hepatitis C compared with patients with histologically more advanced hepatitis C.6 However, there is a lack of evidence on whether treating patients with mild hepatitis C is more cost effective than waiting, and only treating those cases that progress to moderate disease. Previous cost effectiveness analyses have been hindered by a lack of data on effectiveness, costs, disease progression, and health related quality of life (HRQOL).15-18
This study measured the costs and effectiveness of providing antiviral treatment in routine clinical practice as part of a pragmatic randomised controlled trial (RCT). This paper uses these data in a model to assess whether antiviral therapy (either interferon or peginterferon combined with ribavirin) is cost effective over the lifetime for patients with mild chronic hepatitis C.
DISCUSSION
The overall results from this study showed that antiviral treatment, either interferon a-2b or peginterferon a-2b combined with ribavirin, is cost effective for patients with mild chronic hepatitis C. This suggests that in general, the most cost effective strategy is to treat patients at a mild stage rather than follow current recommendations and wait and only treat those patients who progress to moderate disease.11-14 There were however some differences in cost effectiveness according to the subgroup of patients under consideration.
The main results are presented for patients aged 40 years at treatment, the mean age of patients entering the UK mild hepatitis C RCT. For these patients with genotype non-1 (2 or 3), providing either interferon a-2b or peginterferon a-2b combined with ribavirin at a mild rather than a moderate stage was highly cost effective. For patients aged 40 years with genotype 1, providing either antiviral treatment at a mild rather than a moderate stage was associated with a smaller gain in QALYS and higher additional costs. However, even for patients with genotype 1 the costs per QALY gained from antiviral treatment at a mild stage were below the £ 30 000 ($47 000) per QALY threshold that has been used to decide whether a new intervention is worthwhile.34
Subgroup analysis showed that for older patients (aged 65 years) with genotype 1, the intervention is unlikely to be cost effective as their remaining life expectancy is insufficient to gain enough QALYS from successful treatment. Conversely, for younger patients (aged 40 years) with mild chronic hepatitis C, antiviral therapy for mild hepatitis C leads to sufficient gain in QALYS, even for patients with genotype 1, to be cost effective. The analysis did not use age adjusted SVRs. There is evidence that SVRs decline with age,6 and incorporating this effect would make antiviral therapy for mild hepatitis C appear even more cost effective for younger rather than older patients.
Our results differ from those of previous studies that have found that antiviral therapy is universally cost effective for patients with chronic hepatitis C.8,9,10,15,17,18 Previous studies did not focus on patients with mild chronic hepatitis C and used SVRs from multinational clinical trials designed to assess safety and efficacy rather than effectiveness.8,9,10,15,17,18 This study improved on previous cost effectiveness models by using empirical data on effectiveness, health service costs, HRQOL, and disease transition. The use of these empirical estimates together with sensitivity analyses that fully tested for uncertainty in the input parameters enabled the model to produce relatively robust estimates of cost effectiveness. While some of the parameters were collected as part of the UK mild hepatitis C RCT and related directly to the provision of antiviral therapy in the UK, the model structure would be transferable to other health care systems. Indeed, the sensitivity analysis also suggested that the results were robust to some of the methodological standpoints taken, in particular the choice of efficacy data. The results have been presented in a transparent way, with separate results presented according to genotype and age, to assist with the transferability of the results to other countries.
For the cost effectiveness analysis of peginterferon and ribavirin, the model required data on the proportion of patients having an SVR. For this parameter the only data available were from multinational clinical trials of safety and efficacy,6,7 which were adapted, using the effectiveness data from the UK mild hepatitis C RCT, to estimate the effectiveness of peginterferon -2b and ribavirin in routine clinical practice. Using the SVRs from this study leads to a conservative estimate of the relative cost effectiveness of providing peginterferon a-2b and ribavirin at a mild rather than a moderate stage. Recent trial results have shown that the efficacy of peginterferon a-2a and ribavirin is similar to that for peginterferon a-2b with ribavirin.36 The general conclusion that peginterferon a-2b and ribavirin is cost effective for mild hepatitis therefore also applies to peginterferon a-2a and ribavirin.
Although the findings from this study suggest that providing either antiviral treatment at a mild stage is more cost effective than waiting and only treating those cases who progress to moderate disease, it is less clear whether the additional costs of providing peginterferon rather than interferon at a mild stage are justified. The relative cost effectiveness of providing peginterferon and ribavirin at a mild stage depends on the ceiling ratio used to determine whether or not an intervention is cost effective. It is cost effective to provide peginterferon rather than interferon with ribavirin for mild hepatitis C if the ceiling ratio exceeds £ 33 000 ($52 000) per QALY gained. Providing either interferon or peginterferon with ribavirin for all patients with mild hepatitis C would avoid the need to use a liver biopsy to establish the patient's disease stage. Liver biopsies are costly, invasive, and have a small risk of morbidity and mortality.37
The finding that antiviral therapy is cost effective for patients with genotype 1 relies on HRQOL improving following an SVR. This improvement was only measured in the UK mild hepatitis C RCT over 6-12 months, on a relatively small number of patients. The sensitivity analysis suggested that provided there was any improvement in HRQOL at all following an SVR, then the intervention would be cost effective for patients with genotype non-1. However, previous studies that have recruited more patients and followed them for longer suggest that the improvement in HRQOL may be greater than that observed in this trial.38,39 The cost effectiveness estimates as presented are therefore likely to be conservative.
The main purpose of this study was to estimate whether antiviral therapy was cost effective for patients with mild hepatitis C who met the eligibility criteria for patients included in the UK mild hepatitis C RCT.20 Certain groups were excluded from the study, including patients with human immunodeficiency virus coinfection, ongoing psychiatric morbidity, intravenous drug use, excessive alcohol intake (>28 units for men and >21 units for women), cardiovascular disease, uncontrolled diabetes mellitus, or haemophilia. The choice of parameter estimates in the model reflects this study's target population. In particular, the decision to use general all cause death rates rather than applying a higher all cause death rate for patients with hepatitis C was based on excluding these patient groups.
In the base case analysis, data from the UK tertiary referral centre that assigned most patients to the RCT were used to estimate transition probabilities. The sensitivity analysis suggested that when transition probabilities were taken from the Trent cohort, which may be more representative of disease progression in the UK, the conclusions were unchanged for patients with genotype non-1. However, for patients with genotype 1 using these lower disease transition probabilities meant that the cost per QALY gained exceeded £ 30 000 ($47 000) per QALY. Hence caution should be exercised before applying the findings from the cost effectiveness model to patient groups excluded from the study. Other estimates used in the model, in particular the SVRs following antiviral treatment, may also change if the target population is broadened to include a wider range of patients and centres. Further research is required to establish whether antiviral therapy is effective and cost effective for more general populations of patients with chronic hepatitis C.
To conclude, for patients with chronic hepatitis C, it is generally more cost effective to provide antiviral treatment at a mild rather than a moderate stage, and liver biopsies prior to treatment may no longer be justified. Antiviral treatment at a mild rather than a moderate stage is more cost effective for patients with genotype non-1 than for patients with genotype 1. For patients with genotype 1, the conclusion that antiviral treatment at a mild stage is cost effective depends on the transition probabilities used, the gain in HRQOL following an SVR, and the patient's age. For older patients (aged 65 years or over) with genotype 1, antiviral treatment at a mild stage is not cost effective. The cost effectiveness of treating mild hepatitis C with peginterferon rather than interferon in combination with ribavirin depends on the threshold willingness to pay for a QALY gained.
RESULTS
Base case
Mean (SD) duration of interferon -2b and ribavirin for patients in the mild hepatitis C RCT was 37.8 (15.6) weeks, and mean cost of antiviral therapy was £ 6514 ($10 252) which accounted for 92% of the overall health service costs associated with treatment (£ 7141) (table 2). Mean HRQOL was 0.77 for the mild cases at baseline, 0.66 during treatment, and 0.82 post SVR. Patients at later stages of hepatitis C had higher mean total costs and lower average HRQOL, compared with those with mild disease (table 2).
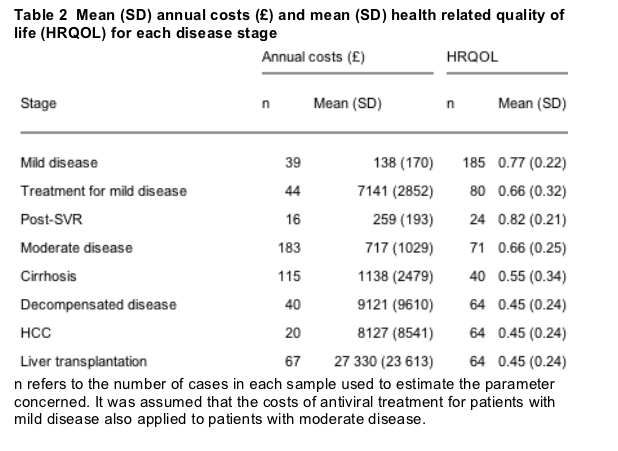
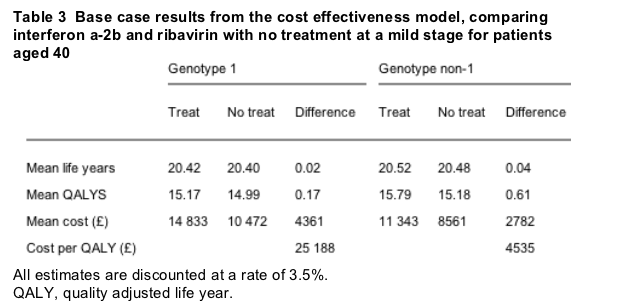
The model found that treating 40 year old chronic hepatitis C patients with interferon a-2b and ribavirin at a mild rather than a moderate stage did not improve life expectancy. Patients treated at a mild stage spent less time with mild or moderate disease, and there was an overall gain of 0.38 QALYS. The higher costs associated with antiviral treatment were not offset by cost savings from preventing patients reaching later disease stages, so the intervention was associated with mean incremental lifetime costs of £ 3647 ($5716) and a mean additional cost per QALY gained of £ 9535 ($14 946).
For patients with genotype 1, treatment at a mild stage was associated with an increase in QALYS of 0.17 and a cost per QALY gained of £ 25 188 ($39 480). For patients with genotype non-1, interferon -2b and ribavirin at a mild stage led to a gain of 0.61 QALYS and a cost per QALY gained of £ 4535 ($7108) (table 3).
Cost effectiveness acceptability curves (fig 2) showed the impact of the uncertainty surrounding the model's input parameters on the cost per QALY gained. At a threshold of £ 30 000 per QALY, the probability that treatment with interferon a-2b and ribavirin at a mild stage was cost effective was 0.86 for 40 year old patients with mild hepatitis C and genotype non-1, and 0.52 for cases with genotype 1.* At this cost per QALY threshold the intervention can be regarded as cost effective for patients aged 20-40 years in either genotype group.
Although patients aged 65 years or over had faster rates of disease progression, their shorter remaining life expectancy meant they generally gained fewer QALYS from antiviral treatment at a mild stage. For patients aged 65 years with genotype 1, treatment with interferon a-2b and ribavirin at a mild stage was not cost effective. For these cases the cost per QALY gained for interferon a-2b and ribavirin was £ 53 017 ($83 099) per QALY and the probability that the intervention was cost effective at £ 30 000 per QALY was 0.28 (fig 2).
Testing methodological assumptions
For patients with genotype non-1, the finding that antiviral treatment at a mild stage was cost effective was robust to making different methodological assumptions. For example, if it was assumed that there was no improvement in HRQOL following a SVR, the cost per QALY gained from antiviral therapy was still well below £ 30 000 per QALY for patients with genotype non-1. Antiviral treatment at a mild stage was still associated with a gain in QALYS as it prevented patients progressing to moderate disease or cirrhosis, health states associated with lower HRQOL than for mild disease. Similarly, early treatment for patients with genotype non-1 remained cost effective if the time horizon for the analysis was reduced from 50 to 30 years (table 4).
The finding that the intervention was cost effective for patients with genotype 1 was sensitive to a number of methodological assumptions. If it was assumed that there was no gain in HRQOL or if the gain was small, then the cost per QALY gained from early treatment exceeded £ 30 000 ($47 000) for patients with genotype 1. Similarly, if disease transition probabilities were taken from a study including a broader range of UK centres (the Trent study), then the cost per QALY gained from treatment at a mild stage rose above £ 30 000. Stopping treatment at 24 weeks for patients with genotype non-1, and using viral kinetics to target treatment according to early response for either genotype group, led to a lower cost per QALY gained for antiviral treatment at a mild stage.
Peginterferon a-2b and ribavirin
The results presented showed that in general it was cost effective to provide antiviral treatment, either peginterferon a-2b or interferon a-2b combined with ribavirin, at a mild rather than a moderate stage (table 5). Although treatment with peginterferon a-2b and ribavirin at a mild stage (strategy D) led to higher costs, it also had the most QALYS, compared with any other treatment option (table 5). The cost per QALY gained from providing peginterferon a-2b and ribavirin at a mild (strategy D) rather than a moderate stage (strategy C) was £ 7821 ($12 259) for patients with genotype non-1 and £ 28 409 ($44 528) for patients with genotype 1.
Assessment of which early treatment option (peginterferon a-2b or interferon a-2b with ribavirin) was the most cost effective depended on the genotype group and the cost per QALY threshold. For patients with mild hepatitis C and genotype non-1, providing peginterferon a-2b (strategy D) rather than interferon a-2b with ribavirin (strategy B), led to a gain of 0.12 QALYS and an additional cost of £ 3741, an incremental cost per QALY gained of £ 32 226 ($50,512). For patients with genotype 1, providing peginterferon -2b (D) rather than interferon a-2b (B) with ribavirin led to a gain of 0.12 QALYS, incremental costs of £ 4064, and an additional cost per QALY gained of £ 32 896 ($51 516). At a cost per QALY threshold of £ 30 000 ($47 000) per QALY, the probability that peginterferon a-2b was cost effective was 0.50 for patients with genotype non-1 and 0.49 for patients with genotype 1.
METHODS
Model
A Markov model was used to estimate the lifetime cost effectiveness of antiviral treatment for patients with mild chronic hepatitis C. The model's structure and main assumptions were similar to previous models for hepatitis C,8,9,10 and have been described previously.19 Briefly, the Markov model required the natural history of the disease to be divided into a series of health states (fig 1). Two hypothetical cohorts with the characteristics of the UK mild hepatitis C trial population (summarised in box 1),20 were entered into the model and faced annual probabilities of progression to subsequent health states (table 1).
Box 1: Base case assumptions
Base case assumptions
* Characteristics of cases at model entry: 40 years old, 60% men, 50% genotype 120
* "Treatment group": mild disease-interferon -2b + ribavirin; cases who do not have a sustained virological response and progress to moderate disease-no treatment
* "No treatment group": mild disease-no treatment; cases who progress to moderate disease-interferon -2b + ribavirin
* Duration of treatment: up to 52 weeks for all patients
* Model duration: up to 50 years
Cases in the "treatment group" were all assumed to have antiviral therapy at a mild stage, with a proportion having a sustained virological response (SVR) and no longer facing a probability of progression.25 Patients in the "no treatment group" did not receive treatment at a mild stage; those cases predicted by the model to reach moderate disease were assumed to have antiviral treatment in accordance with recent UK recommendations.11
Total costs and quality adjusted life years (QALYS) were estimated for each strategy. QALYS are frequently used in cost effectiveness analyses as they recognise that an intervention may have an impact on HRQOL as well as life expectancy. Thus a year of life in "perfect health" would be worth 1 QALY whereas a year of "unhealthy" life would be worth less than 1. HRQOL is commonly recorded using the euriqol (EQ-5D) which asks patients to describe their own HRQOL. The EQ-5D provides estimates of HRQOL on a scale from 0 (dead) to 1 (perfect health). QALYS are then calculated by weighting life expectancy by the HRQOL experienced during each disease stage.
In this study, the total costs and total QALYS were estimated over the cohorts' lifetime which was up to 50 years for patients entering the model aged 40 years (the base case). Cases in the early stages of the model (for example, mild disease) were subjected to general population all cause death rates.24 Cases in the model who reached more advanced stages of liver disease (for example, decompensated disease) were subjected to higher death rates based on estimates from previous studies. These estimates were assumed to encompass deaths related and unrelated to liver disease. Costs and outcomes were estimated over the patient's lifetime so that the full impact of the intervention could be assessed.26
Interventions, effectiveness estimates, and transition probabilities
In the base case, the antiviral therapy considered was interferon a-2ß and ribavirin. Treatment durations and regimens were those used in the treatment arm of the UK mild hepatitis C RCT20; patients randomised to the treatment arm received interferon a-2ß (3 million units thrice weekly) and ribavirin (1000 or 1200 mg daily) for a maximum of 52 weeks. The base case comparator was no treatment at a mild stage which was defined by the monitoring received by the control group in the UK mild hepatitis C RCT and reflected routine practice for mild hepatitis C patients in the UK.20 The UK mild hepatitis C RCT was a pragmatic trial designed to assess the effectiveness of providing the intervention as part of routine clinical practice. The proportions of patients having a SVR following interferon a-2b and ribavirin in the mild hepatitis C RCT were 33% overall, 18% for patients with genotype 1, and 49% for patients with genotype non-1.20 SVR rates were lower than those reported in international clinical trials of interferon and ribavirin for patients with chronic hepatitis C.4,5 SVRs from the McHutchison RCT were used in a sensitivity analysis to assess the robustness of the model's results to the choice of efficacy data.4
The effectiveness data for interferon a-2b and ribavirin from the mild hepatitis C RCT were used as a basis for estimating the likely effectiveness of peginterferon a-2b (1.5 mg/kg) and ribavirin (1000-1200 mg) in routine clinical practice. In an international clinical trial examining efficacy and safety, Manns et al found that the odds ratios of an SVR for peginterferon a-2b and ribavirin compared with interferon a-2b and ribavirin were 1.25 (95% confidence interval 0.70-2.24) for patients with genotype non-1 and 1.43 (1.05-1.96) for patients with genotype 1.6 Based on these odds ratios and proportions having SVRs in the UK mild hepatitis C RCT, SVRs for peginterferon -2b and ribavirin in a routine clinical practice setting were estimated to be 55% for patients with genotype non-1 and 24% for patients with genotype 1.
The model predicted that some patients reached the moderate disease stage. For patients in the "no treatment group", it was assumed that all patients who reached the moderate disease stage had antiviral therapy. The model therefore required SVRs following antiviral therapy for moderate hepatitis C. While Manns et al found that cases with bridging fibrosis or cirrhosis had lower SVRs compared with patients with minimal or no fibrosis,6 there are a lack of data available about whether SVRs are lower for patients with moderate rather than mild disease. The model therefore made the conservative assumption that SVRs following antiviral therapy were the same in patients with mild or moderate disease.
The transition probabilities for mild to moderate disease, and moderate disease to cirrhosis, were estimated by re-analysing cross sectional and longitudinal data21 collected from a UK tertiary referral centre.22 Subsequent transition probabilities were taken from the literature (table 1). The choice of transition probabilities was governed by the model's purpose which was to estimate the cost effectiveness of the intervention in an NHS setting. Here patients are currently required to have attended a hospital for a liver biopsy before treatment. The transition probabilities used in the model were therefore based on studies that recruited patients from a hospital rather than a community setting. The transition probabilities used in this study for progression from mild to moderate disease and moderate disease to cirrhosis were compared with estimates from a recent systematic review of progression rates in hepatitis C.27 The estimates used in our study were lower than those derived from previous studies that have recruited cases from liver clinics (both transition probabilities between 4% and 5%) but higher than estimates from community based studies (between 1.5% and 3% per year).
Costs and HRQOL
Empirical estimates of costs and HRQOL were measured for patients at different stages of hepatitis C and used to populate the model. Each patient included in the UK mild hepatitis C RCT (n = 196) was asked to describe their HRQOL, by completing an EQ-5D questionnaire at each visit during the trial. These health state descriptions were then valued using tariffs derived from a general population survey.28 A health service perspective was taken to costing; the inpatient and outpatient costs incurred from hospital care were included. Detailed resource use data were collected for each patient in the RCT who attended centres in London, Newcastle-upon-Tyne, and Southampton (n = 83).
A separate observational study was used to estimate resource use and HRQOL for patients with moderate disease, cirrhosis, and decompensated cirrhosis.21 Patients were considered for inclusion if they attended any of the three study hospitals listed above for an inpatient admission related to hepatitis C, or for an outpatient appointment at the liver clinic, between 30 March 1998 and 1 April 2000. To identify patients with moderate disease it was necessary to have results from a recent liver biopsy available. A total of 310 cases were identified as having moderate disease; within this group a random sample of 190 cases was taken, and for 183 of these cases the patient's medical records were accessible which meant they were included in the resource use study. Patients were categorised as having cirrhosis according to the results from a liver biopsy or if there was a clinical diagnosis of cirrhosis. A total of 188 cases were identified as having cirrhosis or decompensated cirrhosis, and 175 of these cases had medical records available and were included in the study. Patients were classified as having either cirrhosis (Child Pugh A) (n = 122) or decompensated cirrhosis (Child Pugh B or C) (n = 53) based on the information available from medical records. Hospital resource use attributable to the relevant stage of hepatitis C was then retrospectively recorded from medical records and computer databases.
Unit costs for antiviral therapy and all other medication use were taken from the British National Formulary.29 All other unit costs were collected from the finance departments at the three centres concerned. All costs were reported in 2002-2003 prices (£ ), and the main cost results were converted to US dollars using 2002-3 purchasing power parities to assist with the interpretation of the results.30
To estimate HRQOL, a postal questionnaire including the EQ-5D was sent to the patients included in the costing study. This required patients to describe their HRQOL at the time they completed the questionnaire. The response rates to the questionnaire for patients with moderate disease and cirrhosis were 60% and 54%. At the time of questionnaire completion, six cases were having antiviral treatment and 31 patients had undergone an SVR. The results for the remaining cases with moderate disease (n = 71) or cirrhosis (n = 40) were analysed alongside data from patients in the mild hepatitis C trial (n = 185).
The costs of liver transplantation were taken from a UK study of the costs and outcomes following liver transplantation.31 This study used a similarly detailed approach to cost measurement. This study also collected information on HRQOL using the EQ-5D for patients with decompensated cirrhosis and HCC related to hepatitis C (n = 64); these estimates were used in the cost effectiveness model.
Analysis of costs and cost effectiveness
Total costs were calculated by multiplying each patient's resource use by the relevant unit cost. The model calculated the lifetime costs and QALYS for the treatment and no treatment cohorts, with costs and outcomes discounted at 3.5%.32 Differences in total costs and total QALYS between the treatment and no treatment groups gave the incremental costs per QALY gained. Multivariate Monte Carlo sensitivity analyses were used to consider the random variation across the input parameters and to report cost effectiveness acceptability curves (CEACs).33 CEACs reflect the uncertainty surrounding the cost/QALY from the variation in the input parameters, and report the probability that the intervention is cost effective for different ceiling ratios. The ceiling ratio defines a decision maker's willingness to pay for a unit of health gain. In the UK, it has been suggested that interventions that cost less than £ 30 000 ($47 000) per QALY gained may be regarded as relatively cost effective.34 CEACs therefore make explicit how sensitive the results are to the willingness to pay for improvement in health.
Testing methodological assumptions
In further sensitivity analyses, certain assumptions made in the base case model (box 1) were examined; treatment duration was reduced to a maximum of 24 weeks for patients with genotype non-1, and to 12 weeks for patients identified as having insufficient change in viral load at week 12.20 The impact of assuming different levels of improvement in HRQOL and using a 30 year time horizon was also considered. The base case analysis used data from the UK mild hepatitis C RCT; in the sensitivity analysis, efficacy data from the international RCT by McHutchison et al were used which reported SVRs following interferon a-2b and ribavirin of 28% (genotype 1) and 66% (genotype non-1).4 The base case estimate used early transition probabilities based on data from a single UK tertiary referral centre. The analysis was therefore repeated using transition probabilities based on the Trent study which included seven UK hospitals none of which are tertiary referral centres.35 The mean transition probabilities for mild to moderate disease (0.016) and moderate disease to cirrhosis (0.03) were lower than those used in the base case analysis. Finally, the base case analysis was repeated but using peginterferon a-2b and ribavirin rather than interferon a-2b and ribavirin, with the effectiveness and price of the intervention adjusted accordingly.
|
|
| |
| |
|
|
|