| |
Viramidine Did Not Meet Efficacy Endpoint in Viser2
|
| |
| |
Valeant Pharmaceuticals Reports VISER2 Results for Viramidine(R); Company Initiates Phase 2b Weight-Based Dose-Ranging Study
COSTA MESA, Calif.--(BUSINESS WIRE)--Sept. 12, 2006--Valeant Pharmaceuticals International (NYSE:VRX) today reported summary results of VISER2, the second of two Phase 3 pivotal trials, for Viramidine(R). The company is developing Viramidine (taribavirin hydrochloride), a nucleoside (guanosine) analog prodrug of ribavirin, in oral form, for administration in combination with a pegylated interferon for the treatment of chronic hepatitis C in treatment-naive patients. The VISER2 trial included two co-primary endpoints: one for safety (superiority to ribavirin in the incidence of anemia) and one for efficacy (non-inferiority to ribavirin in sustained viral response, SVR).
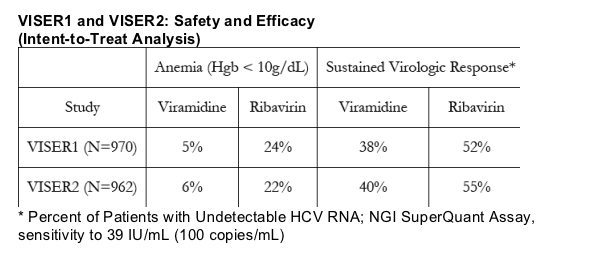
The VISER2 study did not meet the non-inferiority efficacy endpoint on an intent-to-treat (ITT) basis, with overall SVR rates of 40 percent versus 55 percent for the Viramidine and ribavirin arms, respectively. However, consistent with the results seen in VISER1, SVR rates in VISER2 trended higher among patients receiving increased exposure on a mg/kg basis in the Viramidine arm without a substantial increase in the anemia rate.
Consistent with results from the VISER1 trial released earlier in the year, VISER2 confirmed the safety advantages of Viramidine. Anemia rates (Hgb less than 10g/dL) during the treatment period were significantly lower in patients treated with Viramidine than those treated with ribavirin (6 percent versus 22 percent; p less than 0.001).
As a result of the combined VISER1 and VISER2 data, the company also announced that it is initiating a Phase 2b program to evaluate the efficacy of Viramidine at higher doses. The Phase 2b program is a multi-center, randomized, parallel, open-label study in 240 treatment naive, genotype-one patients and will evaluate Viramidine at 20 mg/kg, 25 mg/kg, and 30 mg/kg per day in combination with pegylated interferon alpha-2b. There also will be a control group comprised of ribavirin and pegylated interferon alpha-2b. Treatment duration will be 48 weeks with a post-treatment follow-up period of 24 weeks. Based on a 12-week interim analysis of this study, the company will decide whether to begin a third Phase 3 study at the appropriate higher dose indicated by the Phase 2b study. The company will seek to co-develop the product should it decide to pursue another phase 3 registration trial next year.
Timothy C. Tyson, president and chief executive officer, said, "As expected, VISER2 results were consistent with those seen in VISER1. Although VISER1 and 2 did not meet non-inferiority efficacy endpoints, Viramidine demonstrates meaningful clinical efficacy. Our retrospective analyses of 750 patient plasma samples indicate that Viramidine could be as effective as ribavirin at higher doses. These analyses, coupled with feedback from the medical community that there will continue to be a strong need for ribavirin or ribavirin analogues in the treatment of hepatitis C, indicate that further clinical testing is prudent. Our Phase 2b program should provide us with sufficient information at little relative cost and time to confirm the dose response, select an appropriate dose, test safety at higher doses, establish the path for registration of the drug and make a go/no-go decision."
The company also announced the issuance of a U.S. patent for Viramidine for use in the treatment of hepatitis C, which will not expire until 2020.
The following table summarizes the anemia and SVR rates for both VISER1 and VISER2:
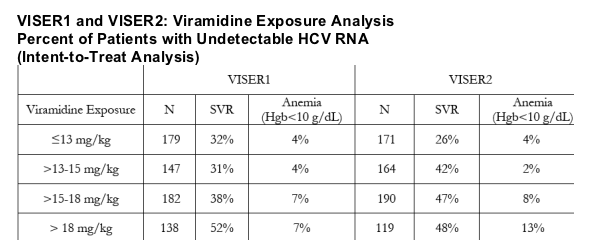
Additional planned analyses of VISER1 and VISER2 data based on weight (mg/kg) indicated that SVR was improved with increased mg/kg concentrations while preserving the safety benefit as summarized below:
The majority of adverse events other than anemia and gastrointestinal side effects were similar between treatment groups. The anemia rate was lower in the Viramidine arm, while the gastrointestinal rate was lower in the ribavirin arm. The most common other adverse events associated with combination therapy included fatigue, headache, insomnia, depression and myalgia.
VISER2 Trial Design
The VISER2 trial (VISER stands for VIramidine's Safety and Efficacy vs. Ribavirin) evaluated a fixed 600 mg BID dose of Viramidine to a weight-based 1,000/1,200 mg daily dose of ribavirin, both in combination with peginterferon alfa 2a. The study, conducted in the United States, Canada, Europe, Israel, Argentina and Australia, enrolled 962 treatment-naive subjects with chronic HCV. Treatment duration was based on genotype, with genotypes 2 and 3 receiving 24 weeks of treatment and genotype non 2, 3 receiving 48 weeks of treatment, each with a post-treatment follow-up period of 24 weeks. The study was stratified for genotype, weight and viral load.
Additional information regarding the Phase 3 trial will be furnished by the company today with the Securities and Exchange Commission on Form 8-K and is also available on the company's Web site at www.valeant.com.
Viramidine is an investigational compound that has not been found by the Food and Drug Administration (FDA) or any other regulatory agency to be safe or effective in the diagnosis, mitigation, treatment or cure of any disease or illness. It may not be sold or promoted in the United States unless and until FDA has approved a New Drug Application. Similar restrictions apply in other countries.
About Valeant
Valeant Pharmaceuticals International (NYSE:VRX) is a global specialty pharmaceutical company that develops, manufactures and markets a broad range of pharmaceutical products primarily in the areas of neurology, infectious disease and dermatology. More information about Valeant can be found at www.valeant.com.
Viramidine is a registered trademark of Valeant Pharmaceuticals International or its related companies. All other trademarks are the trademarks or the registered trademarks of their respective owners.
|
|
| |
| |
|
|
|