| |
28 Days of the Hepatitis C Protease Inhibitor VX-950, in Combination With PEG-Interferon-ALFA-2a and Ribavirin, Is Well-Tolerated and Demonstrates Robust Antiviral Effects
|
| |
| |
Gastroenterology
Volume 131, Issue 3, Pages 950-951 (September 2006)
E.J. Lawitz_, M. Rodriguez-Torres, A. Muir, J. Keane, T. Kieffer, L. McNair, J.G. McHutchison
_ Alamo Medical Research, San Antonio, Texas
Fundacion de Investigation de Diego, Santurce, Puerto Rico
Duke Clinical Research Institute & Division of Gastroenterology, Duke University, Durham, North Carolina
Vertex Pharmaceuticals Incorporated, Cambridge, Massachusetts
ABSTRACT
Background: VX-950 is an orally administered, highly selective peptidomimetic inhibitor of the hepatitis C virus (HCV) NS3-4A protease. Phase 1b studies previously reported have demonstrated that VX-950 was well tolerated alone and with peginterferon-alfa-2a (Peg-IFN) for 14 days, and that the substantial antiviral effects of VX-950 are increased with the addition of Peg-IFN. This study was designed to assess the safety of VX-950 when given in combination with Peg-IFN and ribavirin (RBV) and to evaluate the antiviral response during 28 days of dosing.
Methods:: The VX05-950-102 clinical study included 12 treatment-naive patients infected with genotype 1. All subjects received 750 mg q8h of VX-950, 180 ƒÊg of Peg-IFN weekly, and RBV 1000 or 1200 mg daily. At the completion of 28 days, patients began standard therapy with Peg-IFN/RBV.
Results:
VX-950 + Peg-IFN + RBV was well tolerated, with no serious adverse events and no treatment discontinuations. A detailed analysis of adverse events is in progress.
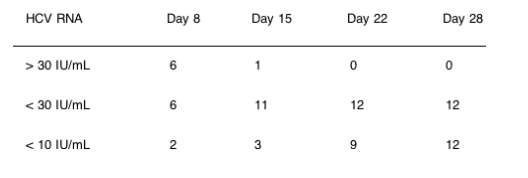
All subjects demonstrated a response to the study drug regimen, with 2 subjects reaching undetectable (< 10 IU/mL, Roche Taqman Assay) levels of plasma HCV RNA within 8 days of the start of dosing, and all subjects HCV RNA-undetectable at the end of the 28-day dosing period.
All subjects showed continual declines in HCV RNA throughout the dosing period, with no subject having viral breakthrough or increase at any time.
Samples were collected for viral sequencing before, during and after dosing; among samples obtained during dosing, only samples Day 2 from some subjects had adequate virus to sequence, and sequencing demonstrated only wild-type virus.
Conclusion: VX-950 + Peg-IFN + RBV was well tolerated for 28 days in patients with HCV genotype 1. The data confirm the rapid and substantial antiviral effects of VX-950, with all subjects achieving undetectable plasma HCV RNA within 28 days of dosing. The combination appears to prevent clinical breakthrough, and to suppress the emergence of NS3 protease inhibitor-resistant variants.
|
|
| |
| |
|
|
|