| |
Colchicine May Delay Progression of Viral Cirrhosis to Liver Cancer
|
| |
| |
Colchicine delays the development of hepatocellular carcinoma in patients with hepatitis virus-related liver cirrhosis
Cancer Oct 2006 Vol 107, Issue 8, 1852-1858
Oscar Arrieta, MD 1 2 *, Jose Luis Rodriguez-Diaz, MD 1, Vanessa Rosas-Camargo, MD 1, Daniela Morales-Espinosa, MD 1, Sergio Ponce de Leon, MD 3, David
Kershenobich, MD 2 4, Eucario Leon-Rodriguez, MD 5
1Department of Medical Oncology, Instituto Nacional de Cancerologia, Tlalpan, Mexico
2Universidad Nacional Autonoma de Mexico, Mexico City, Mexico
3Department of Internal Medicine, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico
4Department of Gastroenterology, Instituto Nacional de Ciencias Medicas y Nutricion, Salvador Zubiran, Mexico
5Department of Hemato-Oncology, Instituto Nacional de Ciencias Medicas y Nutricion, Salvador Zubiran, Mexico
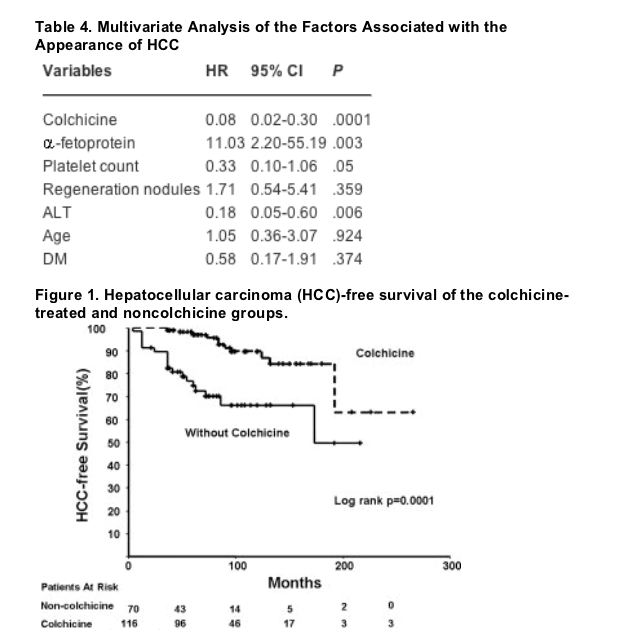
"....The percentage of patients who developed HCC was significantly smaller in the colchicine group compared with the noncolchicine group (9% vs. 29%, P = .0001). The time to develop HCC was significantly longer (in both the univariate and multivariate analyses) (Fig. 1) in patients treated with colchicine, with a mean time of 222 months ± 15 months versus 150 months ± 12 months in the noncolchicine group (P = .0001). The specific survival for HCC was significantly better for the colchicine group (218 months ± 21 months and 252 months ± 11 months; P = .0002). The impact of colchicine remained significant when stratifying the patients by age in the survival analysis (P = .005).... Colchicine may decrease the chronic inflammatory process, providing a protective effect over the development of fibrosis. This protective effect might also arise from the antimitotic action of colchicine, which could reduce the cellular proliferation that results from the inflammatory process, thereby interrupting the hyperplasia-displasia-metaplasia sequence of HCC and preventing mutations leading to HCC..... other randomized studies have failed to demonstrate any beneficial effect on overall mortality, liver-related mortality, and fibrosis progression.....Prospective studies to confirm this observation with a greater number of patients and long-term follow-up may be indicated."
Abstract
BACKGROUND
Hepatocellular carcinoma (HCC) is a malignant neoplasm associated with liver cirrhosis, with an annual incidence of 3% to 9%, which is one of the main causes of death in patients with cirrhosis. Viral hepatitis is associated with an increased risk of HCC, probably due to an inflammatory reaction. Colchicine is an antiinflammatory agent that inhibits the formation of intracellular microtubules, affecting mitosis and fibrogenesis. Diverse clinical studies have failed to demonstrate the benefit of colchicine over the progression of fibrosis in patients with liver cirrhosis; nevertheless, to the authors' knowledge there are no studies that evaluate its effect in the development of HCC.
METHODS
The effect of the administration of colchicine on the development of HCC was evaluated in 186 patients with hepatitis virus-related liver cirrhosis in a retrospective cohort study. The minimum follow-up time was 3 years (median, 84 months ± 2.8 months). One hundred sixteen patients received treatment with colchicine. The characteristics of both groups were similar.
RESULTS
The percentage of patients who developed HCC was significantly smaller in the colchicine group when compared with the noncolchicine group (9% vs. 29%; P = .001). On multivariate analysis, an a-fetoprotein level >/=5 ng/dL (P = .03), a platelet count <100,000 at diagnosis (P = .05), alanine aminotransferase >/=52 IU (P = .006), and a lack of treatment with colchicine (P = .0001) were found to be associated with an earlier development of HCC. The average time for the development of HCC was 222 months ± 15 months and 150 months ± 12 months in the patients who received and who did not receive colchicine, respectively.
CONCLUSIONS
The results suggest that treatment with colchicine prevents and delays the development of HCC in patients with hepatitis virus-related cirrhosis. The protective mechanisms of colchicine over the development of HCC could be related to antiinflammatory properties and inhibition of mitosis. Prospective studies to confirm this observation with a greater number of patients and long-term follow-up may be indicated. Cancer 2006.
Hepatocellular carcinoma (HCC) is responsible for >90% of the primary malignant neoplasms of the liver and is the fifth most common malignant tumor worldwide, responsible for more than 1 million deaths per year.[1] The reported incidence increased by 48% from 1993 to 2000, from 15,000 to 20,200 cases per year in the U.S.[2] Although surgery provides a potential cure, very few patients are good candidates for this procedure due either to the unresectability of the tumor or to the presence of liver failure, worsening both long-term prognosis and overall survival (22% at 1 year).[3]
The major risk factors for the development of HCC are infection by the hepatitis B virus (HBV), hepatitis C virus (HCV),[4] cirrhosis of any etiology, hemochromatosis, other diseases of hepatic metabolism, and exposure to aflatoxins.[5] The annual risk of developing HCC in patients with cirrhosis is 5%, with a published prevalence at autopsy ranging from 7.4% to 23%. HCC is one of the main causes of death in patients with cirrhosis, and as many as 90% of patients with this neoplasm are affected by cirrhosis.[2][6]
The most effective measure to prevent the development of HCC is to avoid exposure to agents that cause cirrhosis. To our knowledge there is no effective treatment available to prevent the development of HCC in patients with cirrhosis, regardless of its etiology. The only way to improve general survival is to detect the disease in the early stages, with close follow-up programs.[6]
To our knowledge, the oncogenic mechanisms involved in the pathogenesis of HCC in patients with hepatitis virus-related cirrhosis have not yet been identified, but it appears that neither HBV nor HCV have a direct effect. Several findings appear to link the development of HCC to the virus-induced inflammatory response, which causes destruction of the hepatocytes and fibrosis.
Colchicine is an alkaloid agent that has been widely used in clinical practice for the treatment of acute gout and other immunologic diseases such as psoriasis, scleroderma, and Behcet disease.[7] The cellular effects of colchicine are attributed to its interaction with microtubules, inhibiting the movement of intracellular granules, as well as some leukocyte functions such as adherence, motility, chemotaxis, expression of molecules of cellular adherence, and lysosome degranulation. The alterations in the formation of microtubules also interfere with all phases of mitosis. Similar to any other antimitotic agent, colchicine inhibits the polymerization of microtubules when used at high doses; at low doses it binds to a specific site at the end of the microtubules, stabilizing microtubule dynamics, and forming a colchicine-tubuline complex. This phenomenon induces conformational changes and modulates the mechanisms responsible for losing and gaining stabilization by guanine tri-phosphate (GTP) or guanine di-phosphate (GDP).[8] In vitro studies have demonstrated that colchicine is capable of inhibiting fibroblast proliferation,[9] and it also delays the microtubule-mediated transport of procollagen[10] and increases collagenase activity.[11]
Colchicine has been demonstrated to have an antifibrotic activity in the liver of mice treated with carbon tetrachloride.[12] At the Instituto Nacional de Ciencias Medicas y Nutricion (National Institute of Medical Sciences and Nutrition [INCMNSZ]) a benefit in survival rates was shown,[13] and is a reason why some patients with cirrhosis are still being managed with this agent.
Several clinical studies have tried to evaluate the effectiveness of colchicine in patients with alcoholic cirrhosis, as well as nonalcoholic cirrhosis.[14-16] The majority of these studies displayed negative results, although some research groups have found some benefit to mortality.[13] The combination of 14 randomized studies that included 1138 patients with different causes of cirrhosis did not demonstrate significant effects of colchicine over global mortality, liver disease-related mortality, progression of fibrosis, ascites, esophageal varices, hepatorenal syndrome, portal vein pressure, and functional status.[14-16] Nevertheless, to our knowledge, there are no studies published to date that have evaluated its effect in the development of HCC.
We investigated the effect of long-term colchicine administration in patients with hepatitis virus-related cirrhosis on the development of HCC in a retrospective cohort study.
MATERIALS AND METHODS
We included patients with hepatitis virus-related cirrhosis treated between January 1980 and January 2000 at the INCMNSZ, with a minimum follow-up period of 3 years. The diagnosis of cirrhosis was established by biopsy or clinical data of chronic liver disease, complications such as portal hypertension, esophageal varices with or without a previous episode of bleeding, splenomegaly, ascites with a previous episode of spontaneous bacterial peritonitis in the absence of other nonhepatic causes, hepatic encephalopathy in the absence of other metabolic causes, and hypoalbuminemia or hyperbilirubinemia in the absence of a known cause of obstruction of the bile duct of at least 1 year after appearance with progressive liver failure.
Three hundred thirty-eight medical records from patients with diagnosis of chronic liver disease with confirmed or suspected cirrhosis were analyzed. The exclusion criteria were: 1) incomplete medical records; 2) patients who did not meet the inclusion criteria or who had alcohol-related cirrhosis; 3) misdiagnosis; 4) a follow-up period of <3 years; 5) the use of immunosuppressants; 6) living donor liver transplant recipient; 7) previous use of immunomodulators such as interferon; and 8) diagnosis of HCC at the first medical visit to the institute. The following demographic, clinical, and biochemical data were collected at the time of diagnosis: age, gender, tobacco use, alcohol consumption, history of diabetes, presence of ascites or hepatic encephalopathy, pharmacologic treatment, serum levels of aspartate aminotransferase, alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase, albumin, -fetoprotein (AFP), prothrombin activity, platelet count, hemoglobin, leukocyte count, and the presence of esophageal varices. The endpoint in this study was defined as the development of HCC.
During the surveillance period, patients were followed with abdominal ultrasonography (US) and determination of AFP levels, physical examination, and routine biochemical tests every 3 months to 6 months. Patients received colchicine based on the treating physicians' medical decision; those who received treatment with colchicine had treatment initiated at the time of the diagnosis of cirrhosis. The dose administered was 1 mg/day 5 days per week (Monday to Friday) for all patients; no patients discontinued the use of colchicine due to adverse effects related to its administration.
Statistical Analysis
For descriptive purposes, continuous variables were summarized as arithmetic means and standard deviation (errors), and categoric variables as relative frequencies and proportions. Inferential comparisons were made using the Student t test or the Mann-Whitney U test according to the distribution of the data (normal and nonnormal) determined by the Kolmogorov-Smirnov test. The chi-square or Fisher exact test were used to test significance between categoric variables. The value was set at P = .05. Survival time was analyzed with the Kaplan-Meier technique and comparisons among subgroups were performed with a log-rank test. All variables were dichotomized for the analysis of survival curves. The time for the development of HCC was measured (from the diagnosis of cirrhosis to the first sign of appearance of HCC). Adjustment for potential confounders was made by stratification of the log-rank analysis and by the use of the Cox proportional hazards regression multivariate analysis. Colchicine exposure was studied as a binary variable and was included in the Cox model. SPSS software (version 10.0; SPSS, Inc., Chicago, IL) and STATA software (StataCorp, College Station, TX) packages were employed to analyze the data.
RESULTS
During the study period, 338 patients were diagnosed with hepatitis virus-related cirrhosis; of these, only 186 met the inclusion criteria. The main causes for exclusion were lack of clinical, laboratory, and histopathologic criteria required for the diagnosis of cirrhosis; cirrhosis related to causes other than viral hepatitis; and the diagnosis of HCC at the initial medical visit to the institute. The average follow-up time was 84 months ± 2.8 months, with a mean age of 52.2 years ± 0.7 years by the time the diagnosis of HCC was made. The median survival of the patients who did not develop HCC was of 226 months with a standard error of 47, and a mean survival of 192 months ± 12 months; whereas in those patients who developed HCC the median survival was 60 months ± 8 months and the mean survival was 79 months ± 13 months from the diagnosis of LC.
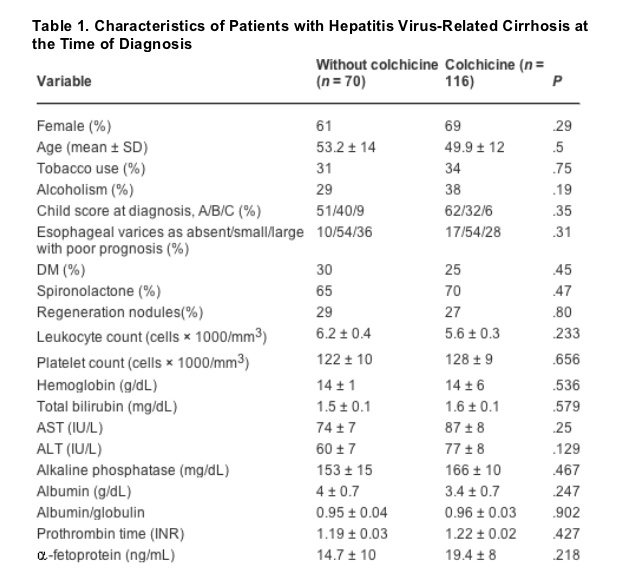
Of the 186 patients who were included in the analysis, 116 received colchicine (62%). The clinical characteristics and laboratory data among both groups of patients at the time of diagnosis of cirrhosis are shown in Table 1. There were no differences noted with regard to gender, age, tobacco use, alcoholism, diabetes, Child-Turcotte-Pugh classification, characteristics of esophageal varices, use of spironolactone, presence of regeneration nodules observed by US, leukocyte count, albumin levels, and levels of AFP. There were no statistically significant differences when both groups of patients were stratified according to their age (X2 3 df = 5.83, P = .12) (Table 2).
After 3 years of follow-up, 9% of the colchicine group presented with an improvement in their Child score, 35% remained stable, and 56% developed disease progression, whereas in the noncolchicine group, 2.5% of patients presented with an improvement in their Child score, 37% remained stable, and 60% developed disease progression (P = .547), thereby demonstrating that colchicine has no direct effect over the progression of cirrhosis as evaluated by the Child score. The median follow-up time in the colchicine group was of 96 months versus 60 months in the noncolchicine group (P = .001, Mann-Whitney U test).
When analyzing the time periods of initiation of treatment with colchicine or lack of it, we did not find any differences between either group (before 1980, 0% vs. 0.86%; 1980-1984, 1.4% vs. 3.45%; 1985-1989, 10% vs. 10.34%; 1990-1994, 41.3% vs. 55.1%; 1995-1999, 42.8% vs. 28.5%; and 2000-2004, 4.29% vs. 1.7%; Fisher exact test, P = .223). The mean time for which the patients in the colchicine group received treatment was of 62.5 months ± 3.9 months (median, 48 months; range, 6-168 months). Ninety-six percent of the patients received treatment for at least 12 months; 87% for at least 24 months; 46% for at least 60 months; and 13% for at least 120 months.
Twenty-seven percent of the patients developed HCC; the diagnosis was made either by biopsy (35%) or by elevation in the AFP level with an identifiable liver tumor (65%). The average time between the diagnosis of cirrhosis and HCC was 206 months ± 11 months; the mean survival was 10 months ± 3 months, with a median survival of 4 months ± 1 month in the patients who developed HCC after diagnosis. On the univariate analysis, the factors associated with the development of HCC at the diagnosis of were the presence of regeneration nodules, a platelet count /=>/=5 ng/mL (Table 3). The variables found to have borderline statistical significance were age >/=52 years and the presence of diabetes mellitus, which were also included in the multivariate analysis (Table 4). In this type of analysis, the factors related to the development of HCC that remained significant were a platelet count 100,000, an AFP level 5 ng/mL, and ALT levels >/=52 IU.
DISCUSSION
Several studies have analyzed risk factors to develop HCC in patients with cirrhosis.[17-22] On multivariate analysis, 3 variables were found to be associated with HCC (platelet count, AFP level, and ALT level). We found that a platelet count >100,000 cells/mm3 (hazards ratio [HR], 0.33; 95% confidence interval [95% CI], 0.10-1.06) is associated with a decreased risk for developing HCC. These results are similar to those reported in other studies.[17][19][21] A low platelet count might reflect a more severe liver failure and portal hypertension and a longer evolution of the disease. We also found that AFP levels >5 ng/mL at the time of diagnosis were correlated with a greater incidence and an earlier appearance of HCC (HR, 11.03; 95% CI, 2.20-55.19). In some studies, a high basal level of AFP has been associated with HCC[18][19][23]; a level >15 ng/mL was found in 10% of patients with hepatitis virus-related cirrhosis and in only 0.7% of those with alcoholic cirrhosis. Based on these findings, we might think that the basal level of AFP may have a predictive value for the development of HCC only in those patients with viral cirrhosis and not in patients with nonviral cirrhosis.[18] Elevated ALT levels at the time of diagnosis also demonstrated an increased risk for the development of HCC, most likely caused by an important impairment of liver function and greater viral activity.
It is known that the hepatic inflammatory process plays an important role in hepatocarcinogenesis.[24][25] To our knowlegde, the oncogenic mechanisms involved in the pathogenesis of HCC in patients with hepatitis virus-related cirrhosis have not yet been identified. It appears that neither HBV nor HVC play a direct role in this process. Some findings suggest that the development of HCC arises from the inflammatory response, which causes destruction of the hepatocytes, regression, and fibrosis.[26] Previous studies in patients with hepatitis virus-related cirrhosis have demonstrated that the rate of fibrotic activity is a risk factor for the development of HCC.[20] Experimental model studies have demonstrated that amelioration of the inflammatory response may decrease the risk of cancer.[27]
Treatment with colchicine in patients with cirrhosis remains controversial. At our institution, a randomized study demonstrated an increase in overall survival.[13] Adhami and Basho[28] also showed in a randomized study that colchicine can improve overall survival of patients with cirrhosis. Nevertheless, other randomized studies have failed to demonstrate any beneficial effect on overall mortality, liver-related mortality, and fibrosis progression.[14-16] Due to such controversies, colchicine is seldom used to treat patients with cirrhosis. To our knowledge, the current study is the first to evaluate the effect of colchicine on the prevention or delay of development of HCC. We found a significant delay in the time to the development of HCC (222 months ± 15 months vs. 150 months ± 12 months); moreover, HCC was less common in the group of patients treated with colchicine (26% vs. 10%). Colchicine may decrease the chronic inflammatory process, providing a protective effect over the development of fibrosis. This protective effect might also arise from the antimitotic action of colchicine, which could reduce the cellular proliferation that results from the inflammatory process, thereby interrupting the hyperplasia-displasia-metaplasia sequence of HCC and preventing mutations leading to HCC. Colchicine has been demonstrated to produce an arrest of HCC cells in vitro in the G2 and M-phases of the cell cycle and to have a radiosensitizing effect.[29] Age has been found to be a significant factor in the development of HCC in several studies.[17][19] We found that patient age to be a variable of borderline statistical significance. Therefore, we decided to perform a stratified analysis of the patients according to age; no statistically significant differences were found (Table 2). We found there was a longer follow-up period in the patients who received colchicine. The differences with regard to follow-up are mainly due to the fact that the patients in the colchicine group had a delayed development of HCC and therefore a greater survival. It is important to point out that the patients did not develop HCC despite having a longer risk time. In addition, we demonstrated an overall survival benefit by performing an age-stratified analysis of HCC-free survival and colchicine treatment (P = .005).
In conclusion, the results of the current study appear to demonstrate that colchicine can prevent the development of HCC, independent of other factors such as age, platelet count, AFP level, and transaminase levels. Prospective studies to confirm this observation with a greater number of patients and long-term follow-up may be indicated.
|
|
| |
| |
|
|
|