| |
Saliva-based hepatitis C test developed
|
| |
| |
Wagdy Sawahel
23 December 2005
Source: SciDev.Net
Israeli scientists have developed a saliva-based test to detect the hepatitis C virus, and say it could be appropriate for mass screening programmes in developing countries.
Hepatitis C is common in the developing world, but the conventional method of detecting the virus in a blood sample is often inaccessible to poorer nations.
Current tests use a sample of the patient's serum, the liquid part of blood in which blood cells are suspended, and detect antibodies that the body produces in reaction to the virus.
But such tests are costly, complicated and rely on an array of medical equipment and skilled personnel.
Now researchers led by Arieh Yaari of Soroka University Medical Center, Israel, have shown that saliva can be used instead of serum to detect the virus.
They carried out their study on 37 dialysis patients, people without kidney function whose blood must be passed through a machine to filter out waste products.
Such patients have a high incidence of hepatitis C and may resemble ill people in developing countries in their immune response levels.
Yaari and colleagues report 100 per cent success at detecting hepatitis C in the saliva of patients who had symptoms of the disease. This is comparable to the results of testing serum.
In patients who had the virus but had yet to develop symptoms, the saliva test was accurate in 94 per cent of cases, while the conventional serum test detected only 63 per cent of infections.
Yaari's team say that as it is cheap and easy to obtain saliva samples, detecting hepatitis C infections using this technique might be economically and clinically important in developing nations.
They add that as the research involved only 37 patients, a larger study is needed to confirm the results. This could focus on a different high-risk population, for example people in developing countries, say the researchers.
They published their findings online on Monday (19 December) in the Journal of Virological Methods.
Detection of HCV salivary antibodies by a simple and rapid test
Journal of Virological Methods
Volume 133, Issue 1 , April 2006, Pages 1-5
A. Yaaria, , , D. Tovbinb, M. Zlotnickb, M. Mostoslavskyb, Y. Shemer-Avnia, N. Hanukaa, Z. Burbeac, Z. Katzird, S. Storche and M. Margalithf
aDepartment of Virology, Soroka University Medical Center, POB 151, Beer Sheva 84101, Israel
bDepartment of Nephrology, The Soroka University Medical Center, Beer Sheva, Israel
cDepartment of Nephrology, Rambam Medical Center, Haifa, Israel
dInstitute of Nephrology, Edith Wolfson Medical Center, Holon, Israel
eDepartment of Internal Medicine A, Bnai-Zion Medical Center, Haifa, Israel
fDepartment of Virology, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel
Corresponding author. Fax: +972 8 6276215.
Received 16 September 2004; revised 22 September 2005; accepted 30 September 2005. Available online 19 December 2005.
Abstract
Hepatitis C (HCV) is common in developing countries, where blood sampling and expensive sophisticated methods for detection are less available. Hemodialysis patients have high prevalence of HCV and may resemble sick populations in developing countries in relation to immunosuppression and antibodies production. For these reasons anti-HCV antibodies were assayed in saliva of hemodialysis patients by ImmunoComb II assay that is less laborious, relatively inexpensive and easy to perform If the findings are confirmed by larger studies this method may be useful especially in developing countries.
Serum and saliva samples were obtained from 37 hemodialysis patients and assayed by ImmunoComb II kit. In positive PCR patients the saliva test had 100% sensitivity, which was as good as serum anti-HCV Axsym testing. Saliva testing had a similar or better specificity than the serum method.
1. Introduction
Over 170 million people worldwide are infected with HCV, including 4 million Americans and 9 million Europeans (Rizzetto, 1992). In developing countries where resources and facilities may be significantly limited the prevalence HCV is higher as compared to the developed world (Wild and Hall, 2000)
Infection with HCV becomes persistent in ≥70% of infected people and may be associated with chronic hepatitis, cirrhosis and hepatic cell carcinoma (Rizzetto, 1992). The hepatitis C virus was identified in 1989 using a recombinant DNA (cDNA) approach (Choo et al., 1989). Currently, routine diagnosis of HCV is based on detecting antibodies (anti-HCV) in serum by enzyme immunoassay (EIA) (Kuo et al., 1989). However, these methods require venipuncture, sophisticated methods and expensive equipment, which may not be appropriate for mass detection in poor developing countries.
Saliva is easy to obtain, especially in outdoor settings and in children. Thus, detecting infections using saliva samples may be of significant clinical, economical and epidemiological importance.
A correlation between salivary antibodies and serum antibodies has been previously reported in relation to viral infection and immunization. Those viral infections included the human immunodeficiency virus, hepatitis A virus, rubella virus, Epstein-Bar virus and cytomegalovirus (Archibald et al., 1986, Major et al., 1991, Crofts et al., 1991, Stuart et al., 1992, Saleh, 1991, Sarid et al., 2001, Sarid et al., 2002 and Nitsan et al., 1994). Salivary antibodies have also been reported following immunization against poliovirus, rotavirus and hepatitis A virus (Zaman et al., 1991, Ward et al., 1992, Friedman et al., 1993, Hurni et al., 1993 and Laufer et al., 1995).
Previous reports have demonstrated salivary antibodies in HCV seropositive subjects (Elsana et al., 1998, Elsana et al., 2001, Cameron et al., 1999 and Bello et al., 1998).
Hepatitis C is common in hemodialysis patients (Pol et al., 2002), especially in developing countries where isolation techniques and blood donor screening are less feasible (Gul and Iqbal, 2003). Furthermore, this patient population tends to have a certain degree of immunosuppression and impaired antibody production (Descamps-Latscha, 1993), and may thus simulate sick populations in developing countries.
The aim of this study was to compare the detection of the salivary HCV antibodies with the serum antibodies using the relatively inexpensive and easy to perform ImmunoComb II assay. Assessment of anti-HCV antibodies in serum by the Axsym assay, which is routinely implemented, will be used for comparison. If this method proves to be efficient in hemodialysis patients, then it may be tried in populations of developing countries where illness and malnutrition related immunosuppression is prevalent.
2. Materials and methods
2.1. Study population
The study population included 37 chronic HD patients attending the Hemodialysis Unit at the Department of Nephrology at the Soroka University Medical Centre, Beer Sheva, Israel. Initially, patients were classified according to a previous routine serum Axsym test, resulting in 26 (15 males, 11 females, 60 ± 16 years) anti-HCV seropositive patients and 11 (7 males, 4 females, 50 ± 4 years) sero-negative patients. When patients were classified according to PCR the study included 18 sero-positives (10 males, 8 females, 59 ± 14 years).
The study was approved by the institutional Ethics Board and informed consent was obtained from all participants.
2.2. Sample collection and processing
Blood and saliva samples were obtained simultaneously from each patient attending the Unit of Nephrology prior to hemodialysis. Each participant donated two-three blood and saliva samples on different days. Blood samples were collected in sterile tubes, and saliva samples were obtained by asking the participant to spit into a sterile plastic cup. Blood and saliva samples were centrifuged immediately at 3000 rpm, for 15 min at 4 C. The serum was divided into three aliquots in sterile tubes; the cell free fraction of the saliva was divided in two aliquots in sterile tubes. All the aliquots were kept at -70 C until assayed.
2.3. Determination of anti-HCV antibodies by the Axsym assay
One serum aliquot from each patient was thawed at room temperature, diluted to the appropriate dilution with phosphate buffered saline (PBS) pH 7.4 and assayed using the Axsym anti-HCV MEIA third generation (Abbott Diagnostics, Chicago, IL, USA) commercial kit in accordance with the manufacturer's instructions. Because of the high dilution (1:40) performed automatically by the Axsym system we did not test the saliva specimens using this method. The signal/cut off (S/CO) ratio range of less than 10 was defined as low positive anti-HCV, intermediate positive HCV was defined as a S/CO ratio between 10 and 50, and high positive when the S/CO ratio was more than 50.
2.4. Determination of anti-HCV antibodies by ImmunoComb II
The saliva and serum samples were also tested by ImmunoComb II (Orgenics, Yavne, Israel), with a modification in order to enhance the sensitivity of the kit.
ImmunoComb II is a rapid test for the differential detection of anti-HCV antibodies directed against structural (HCV core) and non-structural (NS3 and NS4) viral proteins. The sensitivity of the assay was increased by modifying two steps of the assay procedure according with previous experimental results (Laufer et al., 1995, Elsana et al., 1998 and Bello et al., 1998). The concentration of the saliva specimens were increased by discarding the specimen diluent and the incubation period was prolonged to overnight, at low temperature (4 C), instead 10 min at room temperature. PBS pH 7.4 was used as a diluent. According to the manufacturer's instructions this test is considered as positive when either anti-core or anti-NS3/NS4 HCV antibodies are positive.
2.5. Serum and saliva dilutions
In order to compare the performance of both methods, Axsym and ImmunoComb II, the serum and saliva samples were assayed at several dilutions. Serum specimens were diluted from 1:40 up to 1:10 000. Fig. 1 depicts the results from the different serum dilutions, demonstrating a linear decrease of the Axsym anti-HCV S/CO ratio as dilutions increase, r2 = 0.62, 0.74 and 0.78 for Axsym anti-HCV antibodies, Immunocomb II anti-HCV anti-core and anti-HCV NS3/NS4 antibodies, respectively.
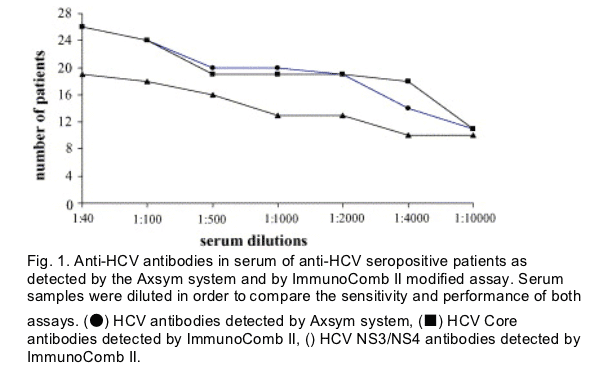
A decrease in anti-HCV positivity as the dilution increases was observed in relation to the anti-core and anti-NS3/NS4 antibodies.
2.6. HCV-RNA detection
Blood samples of all participants in the study were tested for HCV-RNA. RNA was extracted from serum using the HCV specimen preparation kit (Amplicor, Roche Diagnostics, Mannheim, Germany) or the QIAamp blood kit (QIAGEN, GmbH, Hilden, Germany). Reverse transcription (RT) and polymerase chain reaction (PCR) were performed using HCV amplification and detection kits (Cobas HCV amplification and detection, Roche Diagnostica).
3. Results
Twenty-six anti-HCV seropositive patients and 11 seronegative patients (defined by previous Axsym test) were recruited for the present study.
The PCR, Axsym and ImmunoComb II testing results are shown in Table 1. Nineteen serum and saliva samples corresponding to the patients in the middle and the high Axsym S/CO ratio range (>11) were positive for anti-HCV core antibodies. Of these samples, 15 serum and 12 saliva samples were also positive for anti-NS3/NS4 antibodies. Fourteen of those patients were PCR-positive. Thus, it may be concluded that saliva ImmunoComb II HCV testing for core antibodies is more sensitive than for NS3/NS4 antibodies. Both of the two PCR negative patients out of the 17 intermediate and high Axsym S/CO ratio had negative saliva NS3/NS4 testing, suggesting that saliva NS3/NS4 may be more specific than core antibodies testing.
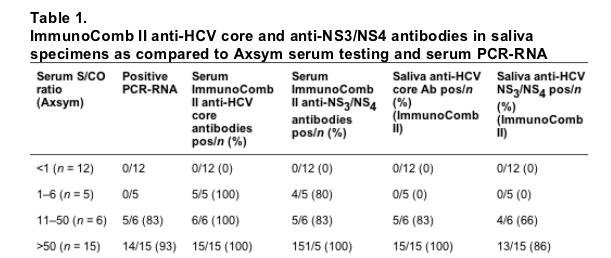
Twenty-one serum and 20 saliva samples corresponding to the patients in the middle and the high Axsym S/CO ratio range (>11) were positive for anti-HCV core antibodies. Of these samples, 20 serum and 17 saliva samples were also positive for anti-NS3/NS4 antibodies. Nineteen of those patients were PCR-positive.
All sera that had been defined as negative for anti-HCV were found anti-HCV negative when tested by Axsym, ImmunoComb II modified assay and by PCR. All saliva samples obtained from those seronegative patients were negative for anti-HCV.
Five out of 26 seropositive patients (19.3%) were anti-HCV low positive for serum by the Axsym assay. These patients had a positive serum ImmunoComb II HCV test, but were negative for serum RNA by PCR and for saliva anti-HCV by the ImmunoComb II.
Six seropositive patients had an intermediate anti-HCV S/CO ratio for serum by Axsym. In these patients one sample was negative for serum RNA by PCR, and ImmunoComb II testing of serum and saliva was positive for all. Fifteen patients had high positive anti-HCV by Axsym. Of these patients one was negative for serum RNA by PCR. All these patients were anti-HCV positive in saliva and in serum when tested by ImmunoComb II.
Patients with active disease, defined as serum positive HCV by PCR, were saliva HCV positive by the ImmunoComb II modified assay, indicating 100% sensitivity, which is as good as detection by Axsym and serum ImmunoComb II testing.
In patients with no active disease, defined as serum RNA negative by PCR, 18 out of 19 (94%) patients were salivary anti-HCV negative and 12 out of 18 (67%) were serum anti-HCV negative by the Axsym and the ImmunoComb II modified assay. Thus, in this group of 26 anti-HCV seropositive hemodialysis patients the saliva ImmunoComb II modified assay test was more specific than and as sensitive as the Axsym system for detecting active HCV.
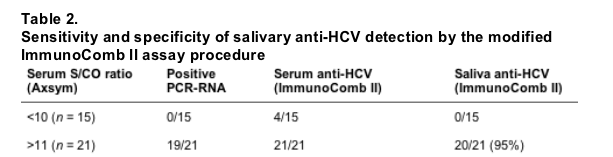
Table 2 shows the results when Axsym S/CO < 10 was considered as false positive. One hundred percent sensitivity of active disease was achieved by intermediate and high anti-HCV Axsym in serum and saliva ImmunoComb II. Specificity of saliva ImmunoComb II HCV was 94% (18/19), similar to the specificity of the Axsym, and 63% of serum ImmunoComb II.
4. Discussion
The aim of the present study was to develop a rapid, sensitive, economical and easy to perform test for the detection of anti-HCV antibodies in saliva.
The main findings of this study are that all patients with active disease, defined as positive by PCR, were positive by saliva ImmunoComb II testing. Thus, saliva ImmunoComb II testing in this cohort of patients had 100% sensitivity, which was as good as the detection serum anti-HCV by the Axsym (S/CO > 10) and serum ImmunoComb II testing.
In patients with no active disease, defined as PCR negative, saliva ImmunoComb II testing had 94% specificity which was superior to serum ImmunoComb II testing and serum Axsym testing (S/CO ≥ 1), both with 63% sensitivity.
When low Axsym anti-HCV (S/C < 10) were considered as false positive (in this group of patients all had negative serum RNA), positive Axsym testing (S/CO > 10) had similar specificity as the saliva ImmunoComb II HCV testing. Thus, in this small group of 37 hemodialysis patients, saliva ImmunoComb II testing was as sensitive for detecting active HCV disease as serum Axsym and had similar or better specificity than the latter method. The saliva ImmunoComb II test has the great advantages of no need for venipuncture, sophisticated equipment and skilled medical personal, as well as being more economical and labor saving. However, it is noteworthy that in our small number of patients, there were no false negatives for Axsym or ImmunoComb II testing. It may be very important to find out whether ImmunoComb II will be positive when false negative patients, who have been previously described in dialysis or immunosuppressed patients, are evaluated.
The results of the present study confirm previous findings from our laboratory and others that saliva may be a surrogate for serum for the detection of anti-HCV positivity (Elsana et al., 1998, Elsana et al., 2001, Cameron et al., 1999 and Bello et al., 1998). It was demonstrated a detection of anti-HCV in 90% of patients with serum anti-HCV by a modified EIA testing (Elsana et al., 1998 and Elsana et al., 2001). However, this assay and the other reported assays were expensive and complicated to perform.
When comparing ImmunoComb II testing of saliva, core antibodies were more sensitive than the NS3/NS4 antibodies. This might suggest that the latter are more specific, but in such a small number of patients such a conclusion is impossible. From a clinical and epidemiologic frame of reference this kit is important for the detection of high risk patients, and using the core antibody seems essential. Future studies with larger numbers of patients may assess the relative contribution of each of those antibodies testing. The ImmunoComb II modified procedure already contains both antibody testing possibilities. However, if the NS3/NS4 antibodies are found to be less contributory and omitting them will lower costs, assessing only core antibodies may be considered for screening tests.
The sensitivity of ImmunoComb II anti-HCV Core and anti-NS3 and -NS4 is in line with previous reports. Alvarado and Leroux-Roels (1999) showed evidence that HCV core, NS3 and NS4 are the most immunogenic antigens. Beld et al. (1999) reported that individuals infected with HCV genotype 1 (in Israel the predominant genotype is 1b) have significantly higher median antibody responses to core and NS4 as compared with those infected with other genotypes. Raghuraman et al. (2003) found that core and NS5 antigens are the most immunogenic.
The implementation of a non-invasive method such as saliva collection is easy and less expensive to perform than venipuncture, and can even be done by unskilled personnel. Saliva is easy to obtain from young children and babies as well. Van Doornum et al. (2001) did not find any statistical difference between the sensitivity and specificity of the salivary anti-HCV testing using two different collecting systems. Thus, we did not use any special device for saliva collection.
In conclusion, the ImmunoComb II modified kit is applicable for anti-HCV screening of saliva in hemodialysis patients and possibly other high risk populations. According to our findings, the subjects with positive salivary antibodies will most probably be those with active disease. This study included a small number of patients of which only 48% were HCV-PCR positive, and no false HCV-negative patients with positive PCR. Thus, this study has to be extended to a larger number of patients that will also include more positive PCR patients as well as false negative anti-HCV patients. Furthermore, a study of anti-HCV antibodies in saliva is worthwhile to perform in other high risk populations, especially in developing countries.
References
Alvarado and Leroux-Roels, 1999 E.C. Alvarado and G. Leroux-Roels, Hepatitis immunology, Rev. Invest. Clin. 51 (1999), pp. 315-322.
Archibald et al., 1986 D.W. Archibald, L.I. Zon, J.E. Groopman, J.S. Allan, M.F. McLane and M.E. Essex, Salivary antibodies as a means of detecting human T cell lymphotropic virus type III/lymphoadenopathy-associated virus infection, J. Clin. Microbiol. 24 (1986), pp. 873-875.
Beld et al., 1999 M. Beld, M. Penning, M. van Putten, V. Lukashov, A. van den Hoek, M. McMorrow and J. Goudsmit, Quantitative antibody responses to structural (Core) and non-structural (NS3, NS4 and NS5) hepatitis C virus proteins among seroconverting injecting drug users: impact of epitope variation and relationship to detection of HCV RNA in blood, Hepatology 29 (1999), pp. 1288-1298.
Bello et al., 1998 P.Y. Bello, C. Pasquier, P. Gourney, J. Puel and J. Izopet, Assessment of a hepatitis C virus antibody assay in saliva for epidemiological studies, Eur. J. Clin. Microbiol. Infect. Dis. 17 (1998), pp. 570-572.
Cameron et al., 1999 S.O. Cameron, K.S. Wilson, T. Good, J. McMenamin, B. McCarron, A. Pithie and R. Fox, Detection of antibodies against hepatitis C virus in saliva: a marker of viral replication, J. Viral Hepatitis 6 (1999), pp. 141-144.
Choo et al., 1989 Q.L. Choo, G. Kuo, A.J. Weiner, L.R. Overby, D.W. Bradley and M. Houghton, Isolation of a cDNA clone derived from a blood-borne non-A non-B viral Hepatitis genome, Science 244 (1989), pp. 359-362.
Crofts et al., 1991 N. Crofts, S. Nicholson, P. Coghlan and I.D. Gust, Testing of saliva for antibodies to HIV-1, AIDS 5 (1991), pp. 561-563.
Descamps-Latscha, 1993 B. Descamps-Latscha, The immune system in end stage renal disease, Curr. Opin. Nephrol. Hypertens. 2 (1993), pp. 883-891.
Elsana et al., 1998 S. Elsana, E. Sikuler, A. Yaari, Y. Shemer-Avni, M. Abu-Shakra, D. Buskila, P. Katzman, L. Naggan and M. Margalith, HCV antibodies in saliva and urine, J. Med. Virol. 55 (1998), pp. 24-27.
Elsana et al., 2001 S. Elsana, E. Sikuler, A. Yaari, Y. Shemer-Avni and M. Margalith, Salivary HCV-antibodies: a follow-up cohort of liver disease patients, Clin. Lab. 47 (2001), pp. 335-338.
Friedman et al., 1993 M.G. Friedman, B. Segal, R. Zedaka, B. Sarov, M. Margalith, R. Bishop and R. Dagan, Serum and salivary responses to oral tetravalent reassortant rotavirus in newborns, Clin. Exp. Immunol. 92 (1993), pp. 194-199.
Gul and Iqbal, 2003 A. Gul and F. Iqbal, Prevalence of hepatitis C in patients on maintenance haemodialysis, J. Coll. Phys. Pak 13 (2003), pp. 15-18.
Hurni et al., 1993 W.M. Hurni, D.S. Laufer, W.J. Miller, J. Ryan and B. Watson, Anti-hepatitis A in the general population and in hepatitis A vaccines using saliva and serum as diagnostic media, Ann. N.Y. Acad. Sci. 694 (1993), pp. 289-292.
Kuo et al., 1989 G. Kuo, Q.L. Choo, H.J. Alter, G.L. Gitnick, A.G. Redecker, R.H. Purcell, T. Miyamura, J.L. Dienstag, M.J. Alter, C.E. Stevens, G.E. Tegtmeyer, F. Bonino, M. Colombo, W.E. Lee, C. Kuo, K. Berger, J.R. Shuster, L.R. Overby, D.W. Bredley and M. Houghton, An assay for circulating antibodies to major etiologic virus of human non-A non-B hepatitis, Science 244 (1989), pp. 362-364.
Laufer et al., 1995 D.S. Laufer, W.M. Hurni, B. Watson, W.J. Miller, J. Ryan and L. Brown, Saliva and serum as diagnostic media for antibody to hepatitis A virus in adults and in individuals who have received an inactivated hepatitis A vaccine, Clin. Infect. Dis. 20 (1995), pp. 868-881.
Major et al., 1991 C.J. Major, S.E. Readm, R.A. Coates, A. Francis, B.J. McLaughlin, M. Wilson, F. Shepherd, M. Fanning, L. Calzavara and D. MacFadden, Comparison of saliva and blood for human immunodeficiency virus prevalence testing, J. Infect. Dis. 163 (1991), pp. 699-702.
Nitsan et al., 1994 C. Nitsan, E. Fuchs and M. Margalith, Antibodies to HIV-1 and to CMV in serum and urine of infected individuals, Aids Res. Retrovir. 10 (1994), p. S 98.
Pol et al., 2002 Pol, S., Vallet-Pichard, A., Fontaine, H., Lebray, P., 2002. HCV infection and hemodialysis.
Raghuraman et al., 2003 S. Raghuraman, T. Subramaniam, D. Daniel, G. Sridharan and I. Abraham, Occurrence of false positives during testing for antibodies to hepatitis C virus among volunteer blood donors in India, J. Clin. Microbiol. 41 (2003), pp. 1788-1790.
Rizzetto, 1992 M. Rizzetto, Viral hepatitis, introduction. In: N. McIntyre, J.P. Benhamou, J. Bicher, M. Rizzeto and J. Rodes, Editors, Oxford Textbook of Clinical Hepatology, Oxford Medical Publication (1992), p. 529.
Saleh, 1991 L.H. Saleh, The use of saliva for the detection of IgG and antibodies against rubella virus: comparison of indirect ELISA and antibody immunoassay, J. Egypt. Public Health Assoc. 66 (1991), pp. 123-134.
Sarid et al., 2001 O. Sarid, A. Anson, A. Yaari and M. Margalith, Epstein-Barr virus specific salivary antibodies as related to stress caused by examinations, J. Med. Virol. 64 (2001), pp. 149-156.
Sarid et al., 2002 O. Sarid, A. Anson, A. Yaari and M. Margalith, Human cytomegalovirus salivary antibodies as related to stress, Clin. Lab. 48 (2002), pp. 297-305.
Stuart et al., 1992 J.M. Stuart, F.A. Majeed, K.A. Cartwright, R. Room, J.V. Parry, K.R. Perry and N.T. Begg, Salivary antibody testing in a school outbreak of hepatitis A, Epidemiol. Infect. 109 (1992), pp. 161-166.
Van Doornum et al., 2001 G.J.J. Van Doornum, A. Lodder, M. Buimer, E.J.C. Van Ameijden and S. Bruistein, Evaluation of hepatitis C antibody testing in saliva specimens collected by two different systems in comparison with antibody and anti-HCV-RNA in serum, J. Med. Virol. 64 (2001), pp. 13-20.
Ward et al., 1992 R.L. Ward, K.A. Pax, J.R. Sherwood, E.C. Young, G.M. Schiff and D.I. Bernstein, Salivary antibody titers in adults challenged with human rotavirus, J. Med. Virol. 36 (1992), pp. 222-225.
Wild and Hall, 2000 C.P. Wild and A.J. Hall, Primary prevention of hepatocellular carcinoma in developing countries, Mutat. Res. 462 (2000), pp. 381-393. Abstract | Full Text + Links | PDF (139 K)
Zaman et al., 1991 S. Zaman, B. Carlsson, F. Jalil, L. Mellander, A.L. Van Wezel, M. Bottiger and L.A. Hanson, Comparison of serum and salivary antibodies in children vaccinated with oral live parenteral inactivated poliovirus antigen concentrations, Acta Paediatrica Scand. 80 (1991), pp. 1166-1173.
|
|
| |
| |
|
|
|