| |
STEATOSIS IN CHRONIC HEPATITIS C: WHY DOES IT REALLY MATTER?
|
| |
| |
Gut Jan 2006;55:123-130
T Asselah1, L Rubbia-Brandt2, P Marcellin1 and F Negro3
1 Service d'Hepatologie and INSERM CRB3, Hopital Beaujon, Clichy, France
2 Service de Pathologie Clinique, Hopital Universitaire, Geneve, Switzerland
3 Services de Pathologie Clinique, de Gastroenterologie et d'Hepatologie, Hopital Universitaire, Geneve, Switzerland
.....IN HIV+ individuals, what is the risk of steatosis? Many of the risk factors listed below are present in some HIV+ individuals...insulin resistance, diabetes, elevated lipids.....(note from Jules Levin)
".....HCV core protein is sufficient to induce lipid accumulation in hepatocytes .......It has been shown that the HCV core protein causes mitochondrial injury, leading to oxidative stress....(note from Jules Levin: if so, does HCV exacerbate lipoatrophy?)...... results provide direct experimental evidence for the contribution of HCV in the development of insulin resistance.... Hui and colleagues41 observed that patients with chronic hepatitis C with mild fibrosis (stage 0 or 1) had higher levels of insulin, C peptide, and HOMA-IR (all p0.01) compared with matched healthy controls.... HOMA-IR was an independent predictor for the degree of fibrosis (p<0.001) and the rate of fibrosis progression (p = 0.03).....their findings support the hypothesis that insulin resistance may contribute to fibrosis progression in chronic HCV infection..... In patients with HCV infection, insulin resistance may be one factor responsible for both steatosis in one way and increasing fibrosis in another.... steatosis disappeared when HCV replication was inhibited by treatment and recurred when replication relapsed after treatment withdrawal..... steatosis seems to negatively influence SVR....."

SUMMARY
Hepatic steatosis (fatty deposits in the liver) is a common histological feature of chronic hepatitis C. Various factors are associated with hepatic steatosis, including obesity, high alcohol consumption, diabetes type II, and hyperlipidaemia. These factors may contribute to steatosis in patients with chronic hepatitis C. In humans, hepatitis C virus (HCV) genotype 3 is more commonly associated with steatosis. In vitro studies and the transgenic mouse model have suggested that the HCV core protein (genotype 1) can induce lipid accumulation within hepatocytes.
However, what is the relevance of steatosis in chronic hepatitis C? It seems that in certain populations, steatosis may be associated with fibrosis progression and this may be genotype specific. The mechanisms underlying this association are unknown; neither is it clear whether this holds true for all patients or only a subgroup. Indeed, after antiviral treatment, virus related steatosis disappears whereas the host associated steatosis remains unaffected.
This review describes and discusses the basic and clinical aspects of the relationship between steatosis and progression of fibrosis, and response to treatment in patients with chronic hepatitis C.
INTRODUCTION
HCV is a major cause of chronic liver disease with about 170 million people infected worldwide. The severity of disease varies widely from asymptomatic chronic infection to cirrhosis and hepatocellular carcinoma.
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum from simple steatosis at one end to severe inflammation with extensive fibrosis or cirrhosis at the other. Hepatic steatosis without inflammation is thought to have a good prognosis but non-alcoholic steatohepatitis (NASH) can progress to cirrhosis in a significant proportion of cases.
Hepatic steatosis is also a common histological feature of chronic hepatitis C but may be independently associated with obesity, high alcohol consumption, type II diabetes, and hyperlipidaemia and, when these occur, may contribute to steatosis in patients with chronic hepatitis C. In this review we will focus on the relevance of steatosis in patients with chronic hepatitis C with respect to disease outcome.
FREQUENCY OF STEATOSIS IN CHRONIC HEPATITIS C PATIENTS IN COMPARISON WITH THE GENERAL POPULATION
Hepatic steatosis can develop secondary to obesity, diabetes mellitus, alcohol abuse, protein malnutrition, total parenteral nutrition, acute starvation, drug therapy, carbohydrate overload,1-7 and chronic hepatitis C infection. An excellent review of lipid metabolism has been published elsewhere (fig 1).5 Estimates based on imaging and autopsy studies suggest that approximately 20-30% of adults in the USA and other Western countries have excess fat accumulation in the liver1,2; approximately 10% of these individuals (that is, 2-3% of adults) are estimated to have NASH. Wanless and Lentz found steatosis in 70% of obese and 35% of lean patients in a consecutive autopsy study.8 The prevalence of steatosis in patients with abnormal liver tests (in which known causes of liver disease have been excluded) is 64%; of these 50% had steatosis and 50% had steatohepatitis.9
In chronic hepatitis C patients, the prevalence of steatosis ranges from 40% to 86% (mean 55%) (table 1).10-23 The majority of patients with steatosis (78%) have mild steatosis affecting less than 30% of hepatocytes. Thus steatosis occurs more frequently in patients with chronic hepatitis C (55%) than in the general population (20-30%) of adults in the Western world.2
FACTORS ASSOCIATED WITH STEATOSIS IN CHRONIC HEPATITIS C PATIENTS
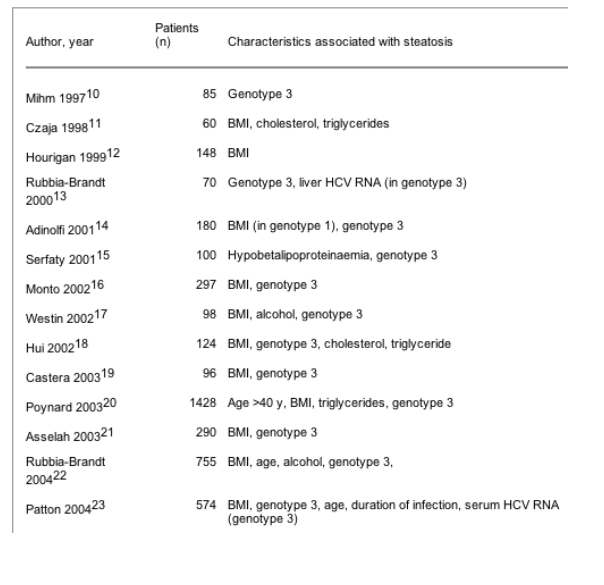
Patients with HCV infection may have coexisting obesity, diabetes, alcohol abuse, etc., which contribute to the development of fatty liver (box 1). In many studies a link between steatosis and a high body mass index (BMI) has been reported (table 2). Adinolfi et al reported that overall, steatosis was not significantly associated with BMI, but when analysis was done by single genotype, a significant association was shown between high visceral fat distribution and grade of steatosis in HCV genotype 1 patients (but not in genotype 3).14 This led to the idea that in patients with HCV infection there is a "metabolic fat" (especially in patients with HCV genotype 1) and a "viral fat" (especially in patients with genotype 3). Epidemiological studies have suggested a link between type 2 diabetes and chronic HCV infection.24-28 However, the presence of confounding factors such as obesity, aging, or cirrhosis precludes the establishment of a definite relationship between these two conditions.
Table 2 Characteristics associated with the presence of steatosis in patients with chronic hepatitis C

STEATOSIS, HCV GENOTYPE 3, AND VIRAL REPLICATION
Several studies have observed a significant association between HCV genotype 3 infection and the presence of steatosis (table 1). In our study, including 290 chronic hepatitis C patients, steatosis, in multivariate analysis, was associated with HCV genotype 3 infection, higher BMI, and a higher grade of necroinflammation.21 Thus steatosis is more frequent in HCV genotype 3 than in HCV genotype 1 infected patients. Steatosis is present in 73% of patients infected with genotype 3 and in 50% of patients infected with genotypes other than 3 (table 1).10-23 The mechanisms underlying this genotype specific steatosis are unknown. We have recently shown that HCV genotype 3 is associated with higher quasispecies heterogeneity than genotype 1.29 Serum levels of apolipoprotein B and cholesterol are reduced in patients in whom steatosis responds to antiviral therapy.15 Thus the disappearance of steatosis correlates with normalisation of apolipoprotein B and cholesterol levels. Hypocholesterolaemia in patients with chronic hepatitis C (especially genotype 3) has been reported by others.20,30 These observations suggest that HCV may interfere with secretion of very low density lipoprotein (VLDL), which is corrected by antiviral therapy.
A significant correlation was observed between steatosis and high titres of intrahepatic negative strand HCV RNA in patients infected with HCV genotype 3, indicating that steatosis may be secondary to a viral cytopathic effect.13 In two studies in patients with HCV genotype 3, an association between grade of steatosis and serum HCV RNA levels was observed.14,20 However, other studies have not confirmed this.31
Experimental models: demonstration that HCV can induce steatosis
In vitro studies and the transgenic mouse model have both suggested that the HCV core protein is sufficient to induce lipid accumulation in hepatocytes. In cell culture, at least two HCV proteins, core and NS5A, are suspected to interact with the cell machinery involved in lipid metabolism.32,33 However, the role of NS5A (if any) seems to be confined to cells coexpressing the HCV core. These proteins interact with apolipoproteins A1 and A2, which are likely involved in triglyceride accumulation and storage in the hepatocytes. Both the core and NS5A proteins are localised on the surface of lipid droplets. Overexpression of core protein further stimulates the formation of lipid droplets.
In transgenic mice it has been reported that HCV core protein inhibits microsomal triglyceride transfer protein (MTP) activity.34 As this is a rate limiting enzyme playing a key role in the VLDL assembly, the direct and likely consequence of its inactivation is accumulation of unsecreted triglycerides, hence steatosis.34 Direct interaction with MTP is unlikely as it would require secretion of the core protein into the endoplasmic reticulum lumen which has not been reported. However, MTP inhibition may still be indirect. One disadvantage of the mouse model is that mice made transgenic with the HCV core protein have normal apolipoprotein B levels in serum.15 A potential indirect effect of the HCV core on the VLDL secretory pathway may be brought about by the non-specific effect of reactive oxygen species (ROS).35 It has been shown that the HCV core protein causes mitochondrial injury, leading to oxidative stress. These effects were prevented by a mitochondrial electron transport inhibitor.35 Thus it seems that, at least in this model, the core protein of HCV may cause oxidative injury as a result of its localisation and direct toxic effect on mitochondria. Thus HCV transgenic mice have increased sensitivity to oxidative stress, and increased hepatic lipid peroxidation occurs in response to carbon tetrachloride.36 Increased production of ROS may cause peroxidation of membrane lipids and structural proteins (such as those involved in trafficking and secretion).
Thus there is evidence that HCV proteins can cause steatosis in the absence of an immune response. However, all of these models used constructs derived from genotype 1 isolates. For this reason, we have established an in vitro model of expression of the HCV core protein of several different types (from 1 to 5), and our preliminary data show that the core proteins from both genotypes 1b and 3a share the capability of inducing accumulation of triglycerides in Huh-7 hepatoma cells, although genotype 3a is much more efficient.37 These results raise the issue of which factors, other than the HCV core protein, may induce steatosis observed in humans. In particular, the role of other viral proteins should be properly investigated.
DOES STEATOSIS AFFECT THE PROGRESSION OF THE DISEASE?
Limitations of published studies
Published data on the influence of steatosis in chronic hepatitis C have several limitations. Firstly, only limited studies are available, almost all being cross sectional or retrospective. Histological criteria for fatty liver disease may be different and the populations studied may have different risk factors for steatosis. For example, in a study from the USA, mean BMI was 28.5 kg/m2 and 32% of patients were obese,16 whereas in our study (France), mean BMI was 24 kg/m2 and only 8% of patients were obese.21 Moreover, the HCV genotype distribution differs from region to region. For example, genotype 3 is more frequent in Europe (24%) than in the USA (14%).16,22
Experimental models: steatosis and fibrosis development
In animal models, such as the leptin deficient ob/ob mouse or the leptin receptor deficient mouse (db/db) and rat (fa/fa), there is very marked hepatic steatosis but little steatohepatitis or fibrosis.38 Development of steatohepatitis in these models depends on additional factors, such as endotoxin exposure, acute liver injury (for example, ischaemia-reperfusion), alcohol, excess dietary polyunsaturated fatty acids, or aging. A unifying hypothesis envisages that all causes capable of changing the redox equilibrium of the hepatocyte may result in liver inflammation and fibrogenesis activation. For example, ROS may promote hepatic stellate cell activation and collagen fibre deposition.39 Lipid peroxidation products, such as 4-hydroxy-2,3-nonenal, may elicit activation and nuclear translocation of c-Jun N-terminal kinases, upregulate c-Jun and increase AP-1 binding, all of which may lead to procollagen type I overexpression.40 Furthermore, these phenomena are efficiently prevented by antioxidants.39
Direct involvement of HCV in the development of insulin resistance
A prominent mechanism linking steatosis and fibrogenesis is insulin resistance. The molecular cause of insulin resistance, a major factor in the pathogenesis of type 2 diabetes, is unknown. Several papers have suggested an association between chronic hepatitis C and type 2 diabetes.24-28 This is relevant as prolonged hyperglycaemia results in several metabolic changes that are of interest in liver fibrogenesis and recent studies show indeed a close correlation between the degree of insulin resistance and extent of fibrosis.41,42
Recently, it was shown that glucose tolerance was impaired in a mouse model transgenic for the HCV core gene, with plasma glucose levels being higher at all time points, including in the fasting state, although the difference was not statistically significant.43 Transgenic mice exhibited marked insulin resistance, as revealed by the insulin tolerance test, as well as significantly higher basal serum insulin levels. Feeding with a high fat diet led to the development of overt diabetes in transgenic mice but not in control mice. Moreover, there were increased levels of tumour necrosis factor (TNF-) in the liver of HCV core gene transgenic mice. High levels of TNF- have also been observed in human chronic hepatitis C patients.44 TNF- has been shown to induce insulin resistance in experimental animals and cultured cells.45,46 Inhibition of tyrosine phosphorylation of insulin receptor substrate 1 and 2 may be one of the mechanisms by which a high level of TNF- causes insulin resistance.46-48 Thus TNF- was considered to be a predominant cause of insulin resistance in transgenic mice. Administration of an anti-TNF- antibody restores insulin sensitivity.43 These results provide direct experimental evidence for the contribution of HCV in the development of insulin resistance. There are experimental arguments for a direct role of insulin in fibrosis progression in HCV infection. Firstly, insulin receptors were identified on hepatic stellate cells isolated from liver sections from patients with chronic HCV infection.49 Secondly, in hepatic stellate cells, connective tissue growth factor mRNA and protein were significantly increased following incubation with insulin.50
Diabetes per se may lead to increased production of ROS,51 and secondary upregulation of antioxidant genes, glycosylation of low density lipoprotein and other proteins, as well as the formation of advanced glycation end products (AGE).52 Expression of the AGE specific receptor (RAGE) in the liver is restricted to stellate cells, and is upregulated at the time of activation and transition to the myofibroblasts phenotype.53 In genetically obese rodents, characterised by severe steatosis, insulin resistance is promoted by increased activity of IkappaB kinase-B, the activator of nuclear factor B (NFB).54 The latter is a transcription factor that plays a key role in expression of several proinflammation genes. Activation of NFB may occur via oxidative stress mediated signalling involving the p38 mitogen activated protein kinase (MAPK).55 The link between insulin resistance and steatosis is complex, and the exact sequence of events is unclear, although several hypotheses have been made.56 Again, a potential link between severity of steatosis and fibrosis may be secondary to increased formation of reactive oxygen species by steatotic hepatocytes (fig 2).
Human studies: steatosis and fibrosis progression
Factors influencing fibrosis progression in chronic hepatitis C are poorly understood (box 2).57 There is some controversy with regard to the influence of steatosis on the progression of fibrosis. Among 14 studies, 10 found an association between the presence of steatosis and a higher degree of fibrosis (table 3). However, in most of these studies this association was of low statistical significance. Furthermore, the histological scores used were different, multivariate analysis was not always performed, and the study populations were different regarding risk factors for steatosis. Thus one possible explanation accounting for the discrepancies is that steatosis may be a cofactor of accelerated fibrosis progression as a function of its pathogenesis (that is, viral v metabolic).
The fact that obesity has been related to the risk of cirrhosis is an argument for a role of metabolic steatosis in the progression of fibrosis. In several studies, among alcoholics and patients with HCV infection, obesity favoured fibrosis.58-62 Fibrosis can indeed develop in overweight patients that are devoid of any other causes of liver disease.63 However, in this study, the degree of steatosis was not associated with fibrosis, suggesting that a high BMI could be associated with fibrosis through mechanisms other than steatosis.
At least four studies did not find a link between the presence of steatosis and increased fibrosis (table 3). In our study, steatosis, in multivariate analysis, was associated with HCV genotype 3 infection, higher BMI, and high grade of necroinflammation, but was not associated with high degree of fibrosis.21 However, in a recent European study with 755 consecutive chronic hepatitis C patients, steatosis was independently associated with fibrosis, but multivariate analysis on patients divided according to viral genotype (that is, 3 v non-3) showed that steatosis was associated with fibrosis only in genotype 3 infected patients.22 It seems that "non-viral steatosis" could be as benign as NAFLD whereas viral steatosis (genotype 3 induced steatosis) may accelerate fibrosis progression. In another recent study from the USA,23 the opposite was true (that is, some evidence was presented that steatosis may affect fibrosis progression only among patients with genotype 1).
Hepatitis C virus, insulin resistance, and fibrosis
In one interesting study, Hui and colleagues41 observed that patients with chronic hepatitis C with mild fibrosis (stage 0 or 1) had higher levels of insulin, C peptide, and HOMA-IR (all p0.01) compared with matched healthy controls. In the 250 hepatitis C virus patients (fibrosis stages 0-4), independent predictors of HOMA-IR included higher BMI (p<0.001), previous failed antiviral treatment (p<0.001), portal inflammatory grade (p<0.001), and genotype 3 infection (p = 0.01). Genotype 3 had significantly lower HOMA-IR than other genotypes (which were comparable when adjusted for the effects of the remaining independent predictors). HOMA-IR was an independent predictor for the degree of fibrosis (p<0.001) and the rate of fibrosis progression (p = 0.03). HCV may induce insulin resistance irrespective of the severity of liver disease, and this effect seems to be genotype specific. Furthermore, their findings support the hypothesis that insulin resistance may contribute to fibrosis progression in chronic HCV infection. Interestingly, insulin resistance may contribute to fibrosis progression in chronic HCV infection independent of the grade of steatosis. Surprisingly, in this study, HCV genotype 3 had significantly lower insulin resistance than other genotypes.
In another recent study,64 marked steatosis was associated with HCV genotype 3 infection and high BMI. Overweight patients had increased circulating insulin compared with lean patients. More advanced fibrosis was associated with obesity and insulin concentrations. Interestingly, in obese patients with chronic HCV infection, circulating insulin levels increased at an early stage of fibrosis (an argument for a causal role). We should remember that in patients with NAFLD, increasing insulin resistance is associated with more severe disease and diabetes is a risk factor for disease progression.65 Therefore, in overweight patients with HCV infection, increasing circulating insulin levels may be one factor responsible for the association between increasing BMI and fibrosis.
In a retrospective survey, high serum glucose was found to be associated with advanced fibrosis and higher fibrosis progression rate in chronic hepatitis C patients, and was independent of age at infection or duration of infection.66 The profibrogenic impact of glucose intolerance was higher than obesity. High serum glucose has also been independently associated with fibrosis in alcoholic liver disease.67
STEATOSIS AND NECROINFLAMMATION
Several studies found a link between necroinflammation and steatosis (table 3). Necroinflammatory activity in chronic hepatitis C may fluctuate over time.68
There are several mechanisms which may account for the relationship between steatosis and necroinflammation. In vitro studies have shown that the HCV core protein could lead to oxidative stress.35 Moreover, HCV is associated with increased production of cytokines44 that enhance inflammation and lead to increased lipid peroxidation. Furthermore, some patients may have NASH in addition to having chronic hepatitis C. Steatosis could be the consequence of more severe cell injury and necroinflammation rather than the direct cause of worsening fibrosis.
HOW TO RECONCILE ALL OF THESE DATA?
Taking into account all of the published data, it seems that there is an association between steatosis and fibrosis. But does this mean that steatosis is the cause of fibrosis progression? Steatosis could be a marker of necroinflammation and therefore a marker of fibrosis progression as several studies found a significant association between necroinflammation and high stage of fibrosis.16,21,69,70 Steatosis could also be a marker of insulin resistance. In patients with HCV infection, insulin resistance may be one factor responsible for both steatosis in one way and increasing fibrosis in another. Interestingly, we reported recently the rate and risk of cirrhosis in 96 patients infected by HCV during the bone marrow transplantation period. At a median follow up of 15.7 years, 15 patients developed biopsy proven cirrhosis leading to a cumulative incidence of cirrhosis of 24% at 20 years. By multivariate analysis, HCV genotype 3 was associated with risk of cirrhosis.71
DOES TREATMENT IMPROVE STEATOSIS?
We initially reported a patient with HCV genotype 3 infection, recurrent hepatitis after liver transplantation, and massive steatosis, in whom steatosis disappeared when HCV replication was inhibited by treatment and recurred when replication relapsed after treatment withdrawal.72,73 This observation was confirmed by other studies, thus supporting a cytopathic effect of HCV genotype 3. In the first study, Kumar and colleagues74 reported that in patients with HCV genotype 1 (n = 28) there was no change in steatosis after treatment, irrespectively of response; however in those infected with genotype 3 (n = 34), a sustained virological response (SVR) resulted in significantly reduced steatosis (p<0.001) but no change in steatosis among those without an SVR. In another study involving 1428 naive patients, the presence of steatosis was associated with a lower SVR (p<0.001)20 but in patients with an SVR, steatosis was most improved in those with HCV genotype 3. In genotype 3 responders, baseline low serum cholesterol was corrected by treatment (p<0.001). These results have been confirmed by others.23
Interestingly, in a retrospective analysis of 174 patients with chronic hepatitis C, obesity (and not steatosis) was a negative predictor of SVR.75 It could be that obesity causes steatosis and that each independently diminishes SVR. Obesity decreases interferon bioavailability and impairs the immune response to HCV. The mechanisms underlying the influence of steatosis on SVR remain to be determined. An intriguing aspect that arises from these studies is the fact that steatosis seems to negatively influence SVR, as suggested by earlier reports.76,77 This phenomenon seems to be limited to patients with a non-HCV related type of steatosis as the presence of even severe steatosis in patients with genotype 3 was not necessarily associated with a decreased rate of SVR.20
It is interesting to note that interferon and insulin share some factors in signalling transduction, such as p38 MAPK78: whether insulin resistance translates into a relative resistance to interferon remains a matter of speculation.
CONCLUSION
The relationship between HCV and steatosis in terms of fibrosis and disease progression is unclear. HCV genotype 3 is clearly associated with steatosis ("viral steatosis"). It seems that in some patients, steatosis may be associated with fibrosis progression. The mechanisms underlying this association are unknown. The challenge for the clinician in care of patients with chronic HCV infection and steatosis is to be able to differentiate between those with pure steatosis alone and a benign course from those with progressive disease (the same challenge in separating NASH from NAFLD).
There remain many unanswered questions and there is a need for large prospective studies on hepatitis C patients, using multivariate analysis, which take into account confounding factors for steatosis, insulin resistance, fibrosis, and response to treatment. A meta-analysis of individual patient data (the HCV MAID Study) is ongoing to investigate the relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C. Moreover, we need to develop experimental models using different HCV genotypes in order to understand the mechanisms leading to steatosis.
|
|
| |
| |
|
|
|