| |
Biojector Improved Fuzeon ISRs, Ease of Use, with PK The Same
|
| |
| |
"Enfuvirtide plasma levels and injection site reactions using a needle-free gas-powered injection system (Biojector)"
AIDS: Volume 20(5) 21 March 2006 p 719-723
Harris, Mariannea; Joy, Rutha; Larsen, Gerenea; Valyi, Monicaa; Walker, Ekaterinab; Frick, Lloyd Wc; Palmatier, Robin Mc; Wring, Stephen Ac; Montaner, Julio SGa
From the aBritish Columbia Centre for Excellence in HIV/AIDS, Vancouver, British Columbia, Canada
bBioject Inc., Portland, Oregon
cDrug Metabolism and Pharmacokinetics, Trimeris Inc., Morrisville, North Carolina, USA.
Abstract
Objectives: To assess the use of the Biojector B2000 needle-free gas-powered injection system for subcutaneous administration of enfuvirtide in HIV-infected patients and to compare this system with standard needles and syringes with respect to ease of use, severity of injection site reactions (ISR), and enfuvirtide plasma levels.
Design: An observational study among 32 treatment-experienced HIV clinic patients receiving enfuvirtide.
Methods: Adult patients were assessed before and after switching from standard needles to the Biojector for enfuvirtide administration. Patients used the Biojector for up to 24 weeks and rated ease of use from 0 (easy) to 3 (difficult). ISR were graded from 0 to 31 for signs and symptoms (erythema, induration, pruritus, nodules/cysts, ecchymosis), duration of individual lesions, and number of lesions. Plasma was collected pre-dose and 1 h post-dose for enfuvirtide measurement. The high-pressure liquid chromatography with tandem mass spectrometry method used was specific for enfuvirtide over its known plasma metabolite. Wilcoxon rank sum tests were used to compare needle-based and Biojector outcomes.
Results:
The Biojector was rated as being significantly easier to use (P < 0.001) and reduced the occurrence of ISR compared with standard needles (P < 0.001). Enfuvirtide plasma levels were not statistically different between the two administration methods at either pre-dose trough (P = 0.41) or 1 h post-dose (P = 0.74).
Of 24 patients with available assessments for ease of use with both needles and the Biojector, 20 found the Biojector easy (score, 0) and only 11 found the needle system easy to use. Approximately half (13/24) found the Biojector easier to use than standard needles and syringes, and 11 found both systems equally easy, scoring both as 0. Overall, the Biojector was assessed by the patients as significantly easier to use than the standard needle and syringe system (P < 0.001). There were no patients who found the Biojector more difficult to use than standard needles and syringes.
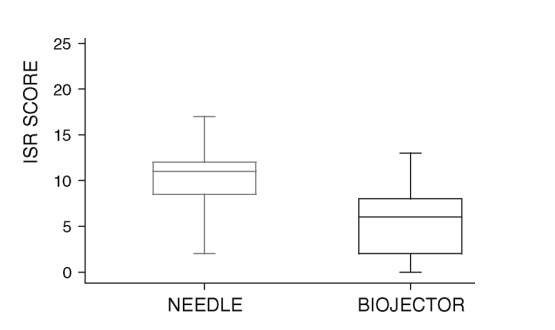
ISR scores were available for 23 patients using both the needle-based system and the Biojector (Fig. 2a). The ISR scores were significantly higher (P < 0.001) for enfuvirtide injections using standard needles [median, 11; interquartile range (IQR), 9-12] than for enfuvirtide injections using the Biojector (median, 5; IQR, 1-8).
There were no significant differences between 20 paired observations of pre-dose trough levels of enfuvirtide by needle and Biojector (P = 0.41), where median enfuvirtide plasma concentration was 1.96 mg/ml (IQR, 1.54-3.47) using needles and 2.56 mg/ml (IQR, 1.86-3.20) using the Biojector (Fig. 2b). Likewise, 1 h post-dose drug levels were the same (P = 0.74) after enfuvirtide administration using standard needles (median, 3.30 mg/ml; IQR, 2.12-4.56) and the Biojector (median, 3.80 mg/ml; IQR, 2.08-4.44) (n = 13).
Conclusions: The Biojector needle-free injection system was easy to use for enfuvirtide administration and was associated with a decreased severity of ISR. Plasma enfuvirtide levels pre-dose and 1 h post-dose were comparable when injecting with standard needles or the Biojector.
Discussion
Enfuvirtide, the first approved fusion inhibitor, has provided a useful treatment option for patients harboring HIV resistant to the other available classes of antiretroviral drugs [1-7]. The drug's main side effect is ISR, which may result in pain and cosmetic concerns, thus potentially hindering adherence and affecting treatment outcomes. Enfuvirtide-associated ISR have been shown to be mitigated by use of the Biojector B2000 needle-free gas-powered injection system.
Patients and caregivers found the Biojector as easy or easier to use than standard needles and syringes. Therefore, self-administration of enfuvirtide using the Biojector system is feasible and acceptable to patients and reduces the severity of ISR, without adversely affecting available plasma levels of enfuvirtide. Of note, this evaluation was performed in a selected group of patients having difficulty with the standard needle-based injection system. Based on these results, further clinical evaluation of the Biojector B2000 for administration of enfuvirtide is warranted in the form of a prospective randomized trial including a wider patient population.
Potential drawbacks of the Biojector are cost and availability. Aside from the device itself (current suggested retail list price US$995), there are costs associated with consumable supplies such as carbon dioxide cartridges and syringes (totaling approximately US$66 per month for twice daily injections), while the supplies needed for standard needle-based injection are included in the purchase price of the drug. The Biojector device has been cleared for marketing in the United States and Canada, and is Conformite Europeene Marked for European distribution, but it is not yet commercially available in Australia and other countries. The Biojector may occasionally be associated with temporary pain or numbness when injection occurs close to a nerve, and some patients still prefer to use standard needles. However, the Biojector may make enfuvirtide an option for patients who refuse to use needles and may enable those who are experiencing significant injection-related problems such as ISR to continue taking enfuvirtide for as long as they are experiencing a therapeutic benefit.
Sponsorship: This study was supported by grants from Roche Canada and Bioject Inc.
Introduction
Enfuvirtide (Fuzeon; Roche, Basel, Switzerland), the first approved HIV fusion inhibitor, has been shown to be a highly effective addition to combination antiretroviral therapy for treatment-experienced HIV-infected patients [1-7]. Studies have shown enfuvirtide to be remarkably free of systemic toxicities [1,2,8].
Enfuvirtide is administered by twice-daily subcutaneous injection using a standard needle and syringe. Local injection site reactions (ISR) are the main side effect observed in studies and clinical use, occurring at least once over 48 weeks in 98% of patients in the TORO trials [8]. While seldom resulting in treatment discontinuation in clinical trials [1,2,8], ISR can be bothersome to patients for a number of reasons, including pain and cosmetic concerns, and may limit their willingness to start or continue on enfuvirtide therapy.
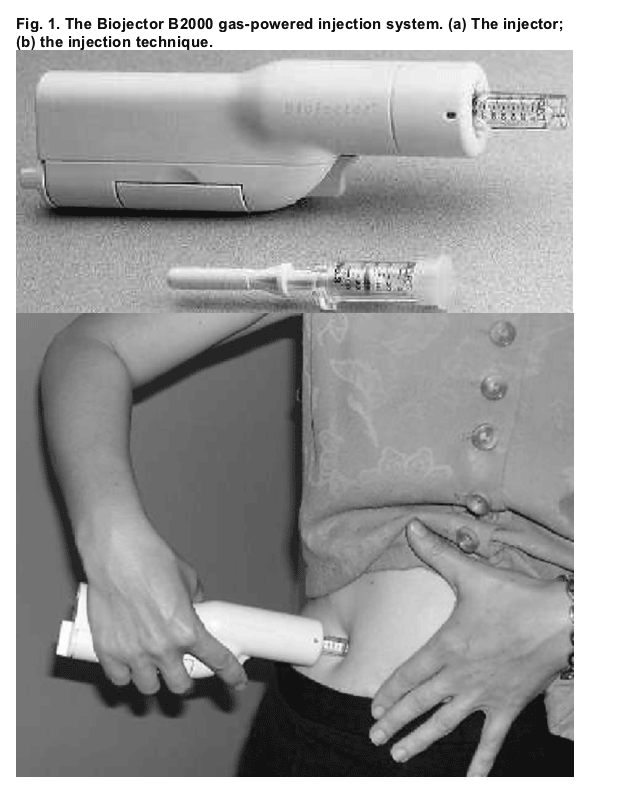
Biojector B2000 (Bioject Inc., Portland, Oregon, USA) is a portable needle-free gas-powered system for subcutaneous injection of medication (Fig. 1). The device forces medication through the skin rapidly in a fine stream that is then dispersed throughout the subcutaneous tissue. Potential advantages of the Biojector compared with standard needles and syringes for administration of enfuvirtide include reduced tissue trauma and local injection pain, thus decreasing the number and severity of ISR. The Biojector may also be easier to use and allow access to more sites for self-injection, because injection can be done with one hand. The time needed to teach patients or caregivers to use the Biojector may be less, and the needle-free system may make enfuvirtide an option for patients who refuse to use needles, for example because of needle-related anxiety or phobia. Furthermore, the absence of needles could make enfuvirtide administration safer for caregivers by reducing the risk of accidental exposure to HIV through needle-stick injury.
The bioequivalence of enfuvirtide plasma levels using the Biojector and standard 27-gauge needles has been demonstrated in a single-dose study. Enfuvirtide plasma concentration-time curves for the two systems were superimposable over the 24 h sampling period [9]. The present study assessed the Biojector for enfuvirtide administration in treatment-experienced HIV-infected patients in the clinic and compared this system with standard needles and syringes with regard to ease of administration, severity of ISR, and enfuvirtide plasma levels.
Methods
Participants
Antiretroviral-experienced HIV-infected adults having demonstrated or anticipated problems with needle-based enfuvirtide treatment and who presented in the clinic between 2 November 2004 and 27 January 2005 were offered a switch to the Biojector after appropriate training from clinic nurses. Patients who were new to enfuvirtide were instructed in the use of standard needles and syringes and used this method for at least 1 week before changing to the Biojector. The enfuvirtide dose was the same for both systems, 90 mg subcutaneously twice daily. All patients were assessed at least once while using needles, and weekly for the first 4 weeks then every 4 weeks while using the Biojector. Assessments included patient evaluation of the ease of use of their current administration system, patient and nurse assessment of the current severity of ISR, and pre-dose and post-dose plasma sampling for enfuvirtide levels. Available follow-up data are presented here to 30 June 2005.
Scoring systems
The ease of use of the administration system was rated by the patient on a scale from 0 to 3: 0, easy; 1, somewhat easy; 2, somewhat difficult; and 3, difficult. The most recent ease of use rating for a given system was used in the analysis.
ISR were scored using a cumulative rating scale based on eight measures of patient response to the administration of enfuvirtide, as used in the TORO studies [1,2]. The first parameter was an overall subjective grading by the patient based on pain and discomfort. The remaining seven parameters were objective ratings of the following signs and symptoms: erythema, induration, pruritus, nodules and cysts, ecchymosis, average duration of individual lesions, and number of individual lesions present. Each of the eight parameters was scored from 0 (none) to 4 (most severe) with the exception of pruritus, which was scored from 0 to 3; this gave a maximum total score of 31. The most recent ISR score with a given system was used for the analysis.
Pharmacokinetics of enfuvirtide
Plasma was collected pre-dose (11-13 h after previous dose) and approximately 1 h post-dose (30-90 min) for enfuvirtide measurement. Bioanalysis employed high-pressure liquid chromatography with tandem mass spectrometry in a method that is specific for enfuvirtide over its known plasma metabolite (enfuvirtide deamidated on the C terminus). The method for assaying enfuvirtide in human plasma has been characterized and shown to be accurate and precise, with inter- and intra-assay accuracy and precision better than ±15% and 15%, respectively, over the calibration range 0.025 to 25.0 mg/ml. The laboratory performing the assay was blinded to the patient identity, administration system (needle or Biojector) and timing (pre- or post-dose) of each sample. Paired observations were collected for both systems of enfuvirtide administration (needle or Biojector) as well as matched observations over time of enfuvirtide levels in patients using the Biojector. To assess enfuvirtide levels over time using the Biojector, plasma was collected from baseline to week 12.
Statistical methods
Non-parametric statistics were used to analyze differences between outcomes of the needle-based and Biojector injection systems. Comparison of ease of use scores, ISR, and enfuvirtide levels were all tested using Wilcoxon signed rank tests for paired observations. Ease of use scores and ISR analyses were tested as one-sided hypotheses where the alternate hypothesis stated the Biojector injector system would be easier to use and be associated with less ISR. Two-sided hypotheses were used for all enfuvirtide pharmacokinetic analyses. All analyses used a significance level of 0.05.
RESULTS
Participants
Between November 2004 and January 2005, 32 HIV-infected adults (29 men and 3 women) were instructed in the use of the Biojector and began using it for enfuvirtide administration. Five of these patients were naive to enfuvirtide, four were on a treatment interruption but had used enfuvirtide in the past, and 23 were receiving enfuvirtide at the time of the switch to the Biojector. The last 23 had received enfuvirtide for a median of 8 months (range, 1 to > 36). The median available follow-up after starting the Biojector was 12 weeks (range, < 1 to 24). Patients were followed until 30 June 2005.
Patient disposition
At the end of the follow-up period, 20 of the 32 patients who started using the Biojector for enfuvirtide administration were still using it, including three who alternated use of the Biojector with standard needles and syringes. Two patients were lost to follow-up, and 10 stopped using the Biojector, for the following reasons. Four discontinued all antiretroviral drugs (one because of fatigue, one because of hepatotoxicity, and two because of virological failure with multidrug-resistant virus); four stopped using the Biojector within the first week because of pain, swelling, and ISR after experiencing similar difficulties with standard needles and syringes. Of these, one went back to using needles, and three discontinued enfuvirtide altogether. Two stopped using the Biojector after more than a month for reasons possibly attributable to the device (both of these patients resumed using standard needles and syringes for enfuvirtide administration). These events were local bruising at the site of injection, which may be related to imperfect injection technique [10], and numbness in the thigh after injecting enfuvirtide at the top of the anterior thigh near the inguinal region, which is not an area recommended for subcutaneous injection [10,11].
In summary, 23 of the 32 patients (72%) who started using the Biojector remained on enfuvirtide at the end of the follow-up period, and 20 (63%) were still using the Biojector to administer enfuvirtide. Only two (6%) discontinued using the Biojector because of adverse events possibly attributable to the device itself.
Ease of use
Of 24 patients with available assessments for ease of use with both needles and the Biojector, 20 found the Biojector easy (score, 0) and only 11 found the needle system easy to use. Approximately half (13/24) found the Biojector easier to use than standard needles and syringes, and 11 found both systems equally easy, scoring both as 0. Overall, the Biojector was assessed by the patients as significantly easier to use than the standard needle and syringe system (P < 0.001). There were no patients who found the Biojector more difficult to use than standard needles and syringes.
Injection site reaction scoring
ISR scores were available for 23 patients using both the needle-based system and the Biojector (Fig. 2a). The ISR scores were significantly higher (P < 0.001) for enfuvirtide injections using standard needles [median, 11; interquartile range (IQR), 9-12] than for enfuvirtide injections using the Biojector (median, 5; IQR, 1-8).
Pharmacokinetics of enfuvirtide
There were no changes detected during the course of therapy for enfuvirtide levels using the Biojector (all pairwise tests; P > 0.1), and, therefore, average enfuvirtide levels over time could be used to compare plasma levels with the needle and Biojector administration methods at pre-dose trough and 1 h post-dose times. There were no significant differences between 20 paired observations of pre-dose trough levels of enfuvirtide by needle and Biojector (P = 0.41), where median enfuvirtide plasma concentration was 1.96 mg/ml (IQR, 1.54-3.47) using needles and 2.56 mg/ml (IQR, 1.86-3.20) using the Biojector (Fig. 2b). Likewise, 1 h post-dose drug levels were the same (P = 0.74) after enfuvirtide administration using standard needles (median, 3.30 mg/ml; IQR, 2.12-4.56) and the Biojector (median, 3.80 mg/ml; IQR, 2.08-4.44) (n = 13).
|
|
| |
| |
|
|
|