| |
Management of chronic hepatitis B and C in HIV-coinfected patients: critical issues
|
| |
| |
Journal of Antimicrobial Chemotherapy May 2006 57(5):815-818
Vincent Soriano*, Pablo Barreiro and Marina Nunez
Department of Infectious Diseases, Hospital Carlos III, Calle Sinesio Delgado 10, 28029 Madrid, Spain
Abstract
One-third of HIV-infected individuals worldwide suffer from chronic hepatitis C virus (HCV) infection, but chronic hepatitis C affects more than 75% of HIV-positive subjects infected parenterally, such as haemophiliacs and intravenous drug users. Chronic hepatitis B virus (HBV) infection, on the other hand, occurs in 10% of HIV-infected persons, coinfection being more prevalent in Southeast Asia. There are two main reasons for considering HCV and HBV therapy as a priority in HIV-coinfected patients: first, the more rapid liver disease progression seen in this population, leading to end-stage liver disease complications, including hepatocellular carcinoma, at younger ages; and second, the higher risk of developing hepatotoxicity following the initiation of antiretroviral therapy in subjects with underlying chronic hepatitis than in HIV-monoinfected individuals. As highly active antiretroviral therapy (HAART) has dramatically improved the prognosis of those with HIV disease, the consequences of associated illnesses such as hepatitis B and C, which are currently among the leading causes of hospital admission and death in the HIV-infected population, have become more relevant. Therefore, the adequate management of viral hepatitis should now be considered a priority in HIV-coinfected patients. Several guidelines have recently been released in response to this demand. In this article, we discuss the most critical issues highlighted in these documents.
Introduction
HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV) share similar routes of transmission, with sexual, parenteral and perinatal transmission being the most frequent modes of acquiring these infections. In contrast, exposure to these viruses is followed by an immune response which differs markedly in its ability to clear the infection. Clearance is maximal for adults exposed to HBV, much lower for HCV and negligible (or non-existent) for HIV. Taking into consideration these two facts, it comes as no surprise that there is a high worldwide prevalence of coinfection with these agents. Figure 1 shows the estimated burden of the population currently living with each of these viruses and the number of coinfected persons.
Hepatitis C
One-third of HIV-infected individuals worldwide suffer from chronic hepatitis C, but HCV affects more than 75% of HIV-positive subjects infected parenterally, such as haemophiliacs and intravenous drug users.1 End-stage liver disease complications have emerged as one of the leading causes of hospital admission and death in HIV-infected patients in developed countries, where the use of highly active antiretroviral therapy (HAART) has halted the progression of HIV-associated immunodeficiency. As a result, classical opportunistic infections are now only rarely seen in HIV-infected individuals in regular clinical care, whereas liver complications due to viral hepatitis coinfections have become more evident.2-4 In the past few years, several guidelines5,6 and reviews7-10 have highlighted this problem and have provided recommendations about how best to manage patients coinfected with HIV and HCV.
Screening for HCV antibodies is key to an effective strategy against hepatitis C, and should be mandatory for all HIV-infected individuals. HCV-seropositive subjects should be tested for serum HCV RNA. Around 15% will have cleared HCV spontaneously. For the rest, quantitative serum HCV RNA measurement using sensitive tests (lower limit of detection in 10-50 IU/mL) and HCV genotyping should be performed before considering any therapeutic intervention against HCV.
The treatment of choice for hepatitis C is a combination of pegylated interferon and ribavirin. Unfortunately, HCV therapy is associated with poorer response and a higher incidence of side effects in HIV/HCV-coinfected patients than in HCV-monoinfected individuals.11,12 However, recent studies suggest that when adequate HCV therapy is administered (using higher doses of ribavirin than in earlier trials, with satisfactory drug compliance and for at least 12 months irrespective of the HCV genotype), and to the most appropriate candidates (excluding active intravenous drug users, alcoholics and subjects with very low CD4 counts), treatment response rates may improve significantly in HIV/HCV-coinfected patients and can approach what is achieved in HCV-monoinfected individuals.13-18 The best coinfected responders are individuals with the following profile: infection with HCV genotypes 2 or 3, low HCV viral load, no cirrhosis, age less than 40 years, elevated ALT concentrations, preserved CD4 counts and low or undetectable plasma HIV RNA. Using the data already available from HCV monoinfection, it is time to design trials in coinfected patients in which therapy is tailored on the basis of individual characteristics. As an example, using variables such as baseline HCV RNA, genotype and week 4 virological clearance, patients could be allowed to complete different lengths of therapy (see Figure 2) in an attempt to balance efficacy and tolerance of the medication.
Figure 2. Preferred treatment duration of HCV therapy taking into account different patient characteristics. Percentages represent estimates of the number of HIV/HCV-coinfected patients in each category in the PRESCO trial. (note from Jules Levin: 24 weeks HCV therapy for genotypes 2/3 has been studied in a limited way and I don't think it is yet firmly established).
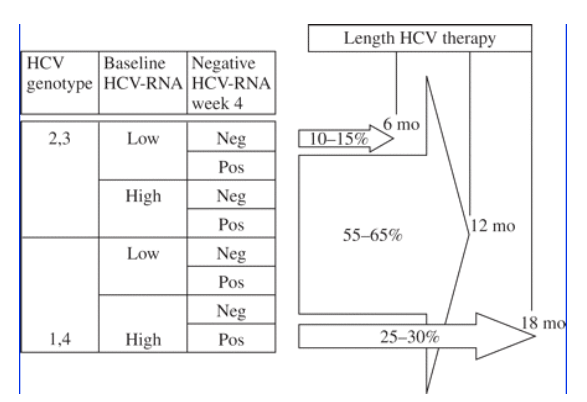
Treatment should be considered early in antiretroviral-naive coinfected patients with stable HIV infection.
In patients already on antiretroviral therapy, HCV therapy should not be administered before ensuring that didanosine is not taken, given the increased risk of mitochondrial toxicities (i.e. pancreatitis and lactic acidosis). If possible, zidovudine should be avoided as well, given the higher risk of anaemia.
Treatment adherence is key to maximizing the chances of success, and side effects of the HCV medication should be managed expertly before discontinuing HCV therapy.
The histological information obtained using either non-invasive procedures (FibroScan, Fibro-test, etc.)19-21 or liver biopsy is useful but these tests should not be considered mandatory before prescribing HCV therapy.22
Progression of liver fibrosis to cirrhosis and hepatocellular carcinoma occurs more rapidly23
,24 and liver toxicity following initiation of HAART is more frequent25,26 in HCV/HIV-coinfected patients. Thus, virological rather than histological findings justify the provision of therapy in this population.22
In patients with evidence of more advanced liver fibrosis, HCV therapy should be considered a real priority
. However, patients with decompensated cirrhosis should not be treated with interferon, given the serious risk of liver failure. On the other hand, in patients with CD4 counts <200 cells/mm3 and/or plasma HIV RNA >100 000 copies/mL, it is advisable to suppress HIV replication and increase the CD4 counts before beginning HCV therapy, as the APRICOT trial showed that side effects of the HCV medication occurred more often in patients with lower CD4 counts.11
Individuals with a history of neuropsychiatric disorders should be seen by an experienced psychiatrist before being considered as candidates for HCV therapy. The psychiatrist may advise about the possibility of interferon-based therapy and/or the usefulness of any co-medication. In patients with mild depression, prophylactic treatment with anti-depressant drugs has proven benefit.10 Subjects who consume a great deal of alcohol and/or are addicted to illegal drugs generally should not be considered suitable for HCV treatment, and medical efforts should concentrate on detoxification.
In summary, liver disease associated with HCV is a growing problem in HIV-positive individuals. The relatively low efficacy of the current medication and its low tolerability should prompt the discovery of new drugs with a direct antiviral activity against HCV. In contrast to antiretroviral drugs, which need to be used indefinitely against HIV, the biology of HCV (which fortunately is not integrated into cellular DNA) provides the chance for limited treatment duration. This opportunity should be much appreciated by both coinfected patients and their doctors. Thus, the provision of HCV treatment should be encouraged without unnecessary delay in the absence of clear contraindication in the coinfected population. A further recent stimulus for the therapeutic intervention is the demonstration of a lack of clinical progression of liver disease in HIV-infected individuals who cleared HCV with therapy: their hepatitis C is cured!27
Hepatitis B
Approximately 10% of the HIV-infected population worldwide suffers from chronic hepatitis B (Figure 1). This figure may approach 20% in Southeast Asia, whereas it is about 5% in North America and Western Europe. Unlike with HCV, infection with HBV is not eradicable and the main goal of therapy is to suppress HBV replication as much as possible and for as long as possible. This translates into histological and clinical benefit. Guidelines for the adequate management of chronic hepatitis B in HIV-coinfected individuals have recently been released,6,28,29 and several reviews30 have updated the knowledge on this topic, providing useful information about how to manage HBV/HIV-coinfected patients.
Four drugs have been approved so far for the treatment of chronic hepatitis B: interferon alpha (standard or pegylated), lamivudine, adefovir and, more recently, entecavir. However, other drugs with anti-HBV activity such as tenofovir and emtricitabine are already approved for the treatment of HIV infection and therefore are frequently used in coinfected patients as anti-HBV agents. In ACTG A5127, the efficacy and safety of tenofovir and adefovir were prospectively compared in 52 HBV/HIV-coinfected individuals, 75% of whom had already failed on lamivudine.31 At 48 weeks, the mean reduction in serum HBV DNA was 3.2 logs in the adefovir arm and 4.4 logs in the tenofovir arm. The study was powered only to show the non-inferiority of tenofovir with respect to adefovir, but the results support the general belief that tenofovir 300 mg/day is much more potent than adefovir 10 mg/day against HBV. The widespread use of tenofovir in HBV/HIV-coinfected patients has demonstrated that HBV can become resistant to tenofovir, although this seems to occur very slowly.32
The prescription of treatment for chronic hepatitis B and the drug of choice are still controversial and may vary in different situations. Four main variables should guide the selection of patients to be treated for HBV and of the drug(s) of choice: transaminase levels, serum HBV DNA viral loads, presence of serum HBV e antigen (HBeAg) and liver fibrosis staging. Given that chronic hepatitis B patients with elevated transaminase levels tend to have liver damage, they should generally be considered primary candidates for treatment (Figure 3), especially in the context of HIV coinfection, since HBV-related liver damage tends to progress faster in HIV-coinfected patients than in HBV-monoinfected patients.33,34 While HBeAg-positive chronic hepatitis B patients tend to show better responses to interferon therapy provided for 6-12 months, HBeAg-negative individuals should preferentially be treated with nucleoside/nucleotide analogues (Figure 4). In HBV/HIV-coinfected patients, a problem arises when no antiretroviral therapy is required but HBV therapy is considered to be necessary. Although some authors have favoured the prescription of adefovir monotherapy in this situation, concern about the selection of the K65R resistance mutation in HIV has precluded this treatment in some cases. Recent data, however, suggest that this risk is negligible.35 In this specific situation, drugs such as entecavir or in the future telbivudine or clevudine, which are potent anti-HBV agents lacking any HIV activity, will likely be the first choice. The 24 week results of the ETV-038 trial were presented at the 2005 Conference on Retroviruses and Opportunistic Infections.36 This trial assessed prospectively the efficacy and safety of entecavir 1.0 mg/day against a placebo in 68 HBV/HIV-coinfected individuals, all of whom had failed prior lamivudine therapy. The virological response was significantly better in the entecavir arm. Even though entecavir acts in patients with lamivudine-resistant HBV, some cross-resistance between these two drugs exists, and therefore the greatest efficacy of entecavir will be obtained in patients without prior exposure to lamivudine.
Figure 3. The HBV treatment decision algorithm.
(Note from Jules Levin: there are varying opinions regarding the level of HBV-DNA that indicates therapy. Older guidelines recommended 100,000 copies/ml as the time to initiate HBV therapy and a target level for HBV-DNA. Recent study data found the risk for HCV & liver disease increases when HBV-DNA is >10,000 copies/ml although the data suggested perhaps 300 copies/ml may be a better target level for HBV-DNA. As of yet I don't think thought leaders have yet to resolve this question and controversy remains.)
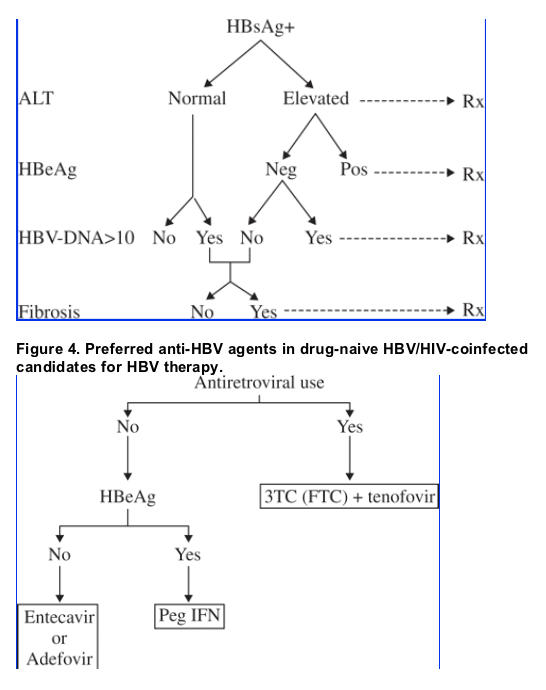
In summary, substantial progress has been made in the treatment of hepatitis B in HIV-positive individuals in recent years. This subset of patients, who typically show more rapid and severe liver damage, now have more treatment options that can be more easily administered and have fewer adverse effects. The achievement and maintenance of HBV suppression (despite the lack of eradication) by judicious use of current HBV therapies will permit prevention of liver complications in most HBV/HIV-coinfected patients.
References
1. Rockstroh J, Mocroft A, Soriano V et al. Influence of hepatitis C on HIV disease progression and response to highly active antiretroviral therapy. J Infect Dis 2005; 192: 992-1002.[CrossRef][ISI][Medline]
2. Martin-Carbonero L, Soriano V, Valencia E et al. Increasing impact of chronic viral hepatitis on hospital admissions and mortality among HIV-infected patients. AIDS Res Hum Retroviruses 2001; 17: 1467-72.[CrossRef][ISI][Medline]
3. Bica I, McGovern B, Dhar R et al. Increasing mortality due to end-stage liver disease in patients with HIV infection. Clin Infect Dis 2001; 32: 492-7.[CrossRef][ISI][Medline]
4. Rosenthal E, Poiree M, Pradier C et al. Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study). AIDS 2003; 17: 1803-9.[CrossRef][ISI][Medline]
5. Soriano V, Puoti M, Sulkowski M et al. Care of patients with hepatitis C and HIV co-infection. Updated recommendations from the HIV-HCV International Panel. AIDS 2004; 18: 1-12.[CrossRef][ISI][Medline]
6. Alberti A, Clumeck N, Collins S et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol 2005; 42: 615-24.[CrossRef][ISI][Medline]
7. Sulkowski M, Thomas D. Hepatitis C in the HIV-infected person. Ann Intern Med 2003; 138: 197-207.[Abstract/Free Full Text]
8. Brau N. Update on chronic hepatitis C in HIV/HCV-coinfected patients: viral interactions and therapy. AIDS 2003; 17: 2279-90.[CrossRef][ISI][Medline]
9. Rockstroh J, Spengler U. HIV/HCV coinfection. Lancet Infect Dis 2004; 4: 437-44.[CrossRef][ISI][Medline]
10. Braitstein P, Palepu A, Dieterich D et al. Special considerations in the initiation and management of antiretroviral therapy in individuals coinfected with HIV and hepatitis C. AIDS 2004; 18: 2221-34.[CrossRef][ISI][Medline]
11. Torriani F, Rodriguez-Torres M, Rockstroh J et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004; 351: 438-50.[Abstract/Free Full Text]
12. Carrat F, Bani-Sadr F, Pol S et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA 2004; 292: 2839-48.[Abstract/Free Full Text]
13. Lindahl K, Stahle L, Bruchfeld A et al. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology 2005; 41: 275-9.[CrossRef][ISI][Medline]
14. Dixit N, Layden-Almer J, Layden T et al. Modeling how ribavirin improves interferon response rates in hepatitis C virus infection. Nature 2004; 432: 922-4.[CrossRef][ISI][Medline]
15. Soriano V, Perez-Olmeda M, Rios P et al. Hepatitis C virus (HCV) relapses after anti-HCV therapy are more frequent in HIV-infected patients. AIDS Res Hum Retroviruses 2004; 20: 351-4.[CrossRef][ISI][Medline]
16. Rendon A, Nunez M, Romero M et al. Early monitoring of ribavirin plasma concentrations may predict anemia and early virological response in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr 2005; 39: 401-5.[CrossRef][Medline]
17. Sherman K, Shire N, Rouster S et al. Viral kinetics in hepatitis C or hepatitis C/HIV-infected patients. Gastroenterology 2005; 128: 313-27.[CrossRef][ISI][Medline]
18. Nunez M, Camino N, Ramos B et al. Impact of ribavirin exposure on early virological response to hepatitis C therapy in HIV-infected patients with chronic hepatitis C. Antivir Ther 2005; 10: 657-62.[ISI][Medline]
19. Ziol M, Handra-Luca A, Kettaneh A et al. Non-invasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41: 48-54.[CrossRef][ISI][Medline]
20. Castera L, Vergniol J, Foucher J et al. Prospective comparison of transient elastography, fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005; 128: 343-50.[CrossRef][ISI][Medline]
21. Myers R, Benhamou Y, Imbert-Bismut F et al. Serum biochemical markers accurately predict liver fibrosis in HIV and hepatitis C virus co-infected patients. AIDS 2003; 17: 721-5.[CrossRef][ISI][Medline]
22. Soriano V, Martin-Carbonero L, Garcia-Samaniego J. Treatment of chronic hepatitis C virus infection: we must target the virus or liver fibrosis? AIDS 2003; 17: 751-3.[CrossRef][ISI][Medline]
23. Martin-Carbonero L, Benhamou Y, Puoti M et al. Incidence and predictors of severe liver fibrosis in HIV-infected patients with chronic hepatitis C: a European collaborative study. Clin Infect Dis 2004; 38: 128-33.[CrossRef][ISI][Medline]
24. Puoti M, Bruno R, Soriano V et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS 2004; 18: 2285-93.[CrossRef][ISI][Medline]
25. Mocroft A, Phillips A, Soriano V et al. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: increased incidence of stopping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Hum Retroviruses 2005; 21:527-36.[CrossRef][ISI][Medline]
26. Nunez M, Lana R, Mendoza J et al. Risk factors for severe hepatic injury following the introduction of HAART. J Acquir Immun Defic Syndr 2001; 27: 426-31.[ISI][Medline]
27. Soriano V, Maida I, Garcia-Samaniego J et al. Long-term follow-up of HIV-infected patients with chronic hepatitis C virus infection treated with interferon-based therapies. Antivir Ther 2004; 9: 987-92.[ISI][Medline]
28. Soriano V, Puoti M, Bonacini M et al. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV international panel. AIDS 2005; 19: 221-40.[ISI][Medline]
29. Brook M, Gilson R, Wilkins E et al. BHIVA Guidelines on HIV and chronic hepatitis: coinfection with HIV and hepatitis B virus infection. HIV Med 2005; 6 Suppl 2: 84-95.[CrossRef][ISI][Medline]
30. Nunez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis 2005; 5: 374-82.[CrossRef][ISI][Medline]
31. Peters M, Anderson J, Lynch P et al. Tenofovir disoproxil fumarate is not inferior to adefovir dipivoxil for the treatment of hepatitis B virus in subjects who are coinfected with HIV: results of ACTG A5127. Abstract 124. 12th Conference on Retroviruses and Opportunistic Infections, Boston, February 2005.
32. Sheldon J, Camino N, Rodes B et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther 2005; 10: 727-34.[ISI][Medline]
33. Colin J, Cazals-Hatem D, Loriot M et al. Influence of HIV infection on chronic hepatitis B in homosexual men. Hepatology 1999; 29: 1306-10.[CrossRef][ISI][Medline]
34. Thio C, Seaberg E, Skolasky R et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the MACS. Lancet 2002; 360: 1921-6.[CrossRef][ISI][Medline]
35. Sheldon J, Corral A, Rodes B et al. Risk of selecting K65R in antiretroviral-naive HIV-infected individuals with chronic hepatitis B treated with adefovir. AIDS 2005; 18: 2036-8.
36. Pessoa W, Gazzard B, Huang A et al. Entecavir in HIV/HBV co-infected patients: safety and efficacy in a phase II study (ETV-038). Abstract 123. 12th Conference on Retroviruses and Opportunistic Infections, Boston, February 2005.
|
|
| |
| |
|
|
|