| |
Genital Human Papillomavirus Infection
|
| |
| |
What about testing the HPV vaccine for men, when will this happen!!!
Clinical Infectious Diseases Sept 1, 2006;43:624-629
Eileen F. Dunne and Lauri E. Markowitz
Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia
"......2 prophylactic HPV vaccines are in, or have completed, phase III clinical trials....Both vaccines have also been shown to have high efficacy among female subjects who have not been exposed to the HPV vaccine types (see discussion below)... The integration of these new strategies and technologies into existing cervical cancer programs may result in decreased morbidity and mortality related to HPV infection in the United States. These strategies may provide even greater benefit in the areas of the world in where there is higher morbidity and mortality related to HPV infection.....Questions remain about the impact and cost-effectiveness of preventing HPV-associated conditions by vaccinating women and men, or by vaccinating women only. The immunogenicity and safety of the quadrivalent vaccine has been similar in young men and women. Although there are potential benefits to vaccinating men, to date, there are efficacy data only for women. The efficacy of an HPV vaccine for anogenital warts and cancers in men is unknown. Further clinical studies will evaluate efficacy in this group. In addition, modeling efforts are underway to evaluate the impact of vaccinating men on outcomes among both men and women...."
Over the past few decades, epidemiology and natural history studies have led to improved understanding of human papillomavirus (HPV) infection and to promising prevention strategies. HPV infection is the cause of anogenital warts and cervical cancer, as well as a proportion of other anogenital and head and neck cancers. Data from clinical trials have resulted in recommendations that support the use of an HPV test in the context of cervical cancer screening and management. Prophylactic HPV vaccine trials have demonstrated high efficacy, and an HPV vaccine that prevents cervical cancer precursors, cervical cancer, and anogenital warts caused by HPV types 6, 11, 16, and 18 was licensed for use in girls and women aged 9-26 years by the US Food and Drug Administration (FDA) in June 2006. In this article, we review genital HPV for the clinician, with a primary focus on the prevalence of HPV infection in the United States.
Human papillomaviruses (HPV) are members of the Papillomaviridae family of DNA viruses. More than 100 types of HPV have been identified; HPV types are primarily differentiated by the genetic sequence of the outer capsid protein L1. Some HPV types cause cutaneous infection (the common skin wart), whereas others cause mucosal infection. There are about 40 types that cause mucosal infection; these types are the focus of this review.
The 40 mucosal HPV types are classified on the basis of their association with cervical cancer. The "low-risk" types of mucosal HPV, also known as "nononcogenic," are associated with anogenital warts, mild cervical dysplasia, and recurrent respiratory papillomatosis; "high-risk" types, also known as "oncogenic," are associated with anogenital cancers and low- and high-grade dysplasias. Eighteen types have been defined as high risk (including types 16 and 18), 12 as low risk (including types 6 and 11), and 3 as indeterminate risk, on the basis of a review of global epidemiological studies [1]. Worldwide, cervical cancers are caused by high-risk HPV: HPV types 16 and 18 account for about 70% of cases [2-4]. A variety of other types account for the remaining 30% of cervical cancers, and these types vary globally. Both the International Agency for Research on Cancer and the National Institutes of Health have concluded that high-risk genital HPV infections act as carcinogens in the development of cervical cancer [5].
The HPV virus is a circular DNA virus with a small genome (about 8 kb). The virus genome has distinct regions that produce neoplastic proteins (E6 and E7), virus production proteins (E1, E2 and E5), and virus capsid proteins (L1 and L2).
HPV infection occurs at the basal epithelium. In the setting of neoplastic progression, HPV DNA may integrate into the host genome. The process of neoplastic transformation-and the factors precluding it-are not completely understood.
Unlike many pathogens, HPV cannot be cultured; infection is identified on the basis of detection of HPV DNA from samples. Treatment is not directed towards HPV itself, but towards the clinical manifestations of infection, including anogenital warts and cervical cancer precursors.
Anogenital HPV infection is estimated to be the sexually transmitted infection (STI) with the highest incidence in the United States; an estimated 20 million people are currently infected, and 6.2 million persons acquire new infection annually [6, 7]. HPV infection is especially common among adolescents and young adults. Prevalence among adolescent girls has been found to be as high as 64% [8] but is usually about 30%, according to many clinic-based prevalence studies [9].
Acquisition of HPV occurs soon after sexual initiation [10, 11]. One study demonstrated that, 48 months after first sexual intercourse, >50% of young women had acquired cervical HPV infection [10]; this study also demonstrated that nonpenetrative sexual activity was associated with HPV acquisition, but much less frequently than with sexual intercourse. Risk factors for HPV infection are primarily related to sexual behavior, including number of sex partners, introduction of new partners, lifetime history of sex partners, and partner's sexual history. In addition, most studies demonstrate that young age (usually characterized as <25 years) is a risk factor for infection. Results of epidemiologic studies are less consistent for other risk factors, including young age at first sexual initiation, inconsistent condom use, parity, dietary factors, genetic factors, smoking, lack of circumcision of male partner, and oral contraceptive use [10-12]. The HPV prevalence in most studies in the United States decreases after age 25, but one cohort study from outside the United States found that there is an increase in prevalence after age 40 [13]. Most HPV prevalence studies have been conducted in girls and women (figure 1); however, available data on the prevalence of HPV among men demonstrate equally high prevalences [14, 15].
Although the incidence of HPV is high, most infections clear. Seventy percent of new infections clear within 1 year, and 91% clear within 2 years [16-18]. It is not known whether clearance indicates elimination of the virus or whether the virus is still present at levels below the limits of detection.
Persistent infection with high-risk types is the most important risk factor for cervical cancer precursor lesions. There is no standard definition of persistence, but it is most commonly defined as detection of the same high-risk HPV types at 2 visits 4-6 months apart. Studies have demonstrated that persistent infection with a high-risk HPV type is associated with a >10-fold risk of high-grade cervical cancer precursors [19, 20].
Serologic testing of HPV is most commonly performed using an ELISA test for antibodies to type-specific virus-like particles (VLPs). Serologic studies of HPV are considered to be research tools; serologic methods are not standardized. Although seropositivity may indicate past or current infection, most persons with HPV infection or persons who develop HPV-associated cancers do not develop antibodies; in fact, as few as 40% of HPV-16 infections are associated with development of HPV-16 antibodies [21, 22]. Serologic assessments in different studies are difficult to compare, because of the varying sensitivity of HPV serologic assays.
CLINICAL CONSIDERATIONS
Most HPV infections result in no clinical changes, although some experts consider detection of any HPV to indicate undetectable changes in the epithelium. The clinical manifestations of HPV infection include anogenital warts, recurrent respiratory papillomatosis, cervical intraepithelial neoplasias (I, II, and III), and cancers, including cervical, anal, vulvar, vaginal, penile, and a subset of head and neck cancers [3, 23-29] (table 1). HPV-16 is the single most common HPV type in anogenital cancer precursor lesions, cancers, and a subset of head and neck cancers [3, 26, 27]. HPV-6 and HPV-11 are the types most frequently responsible for anogenital warts and recurrent respiratory papillomatosis [24, 29].
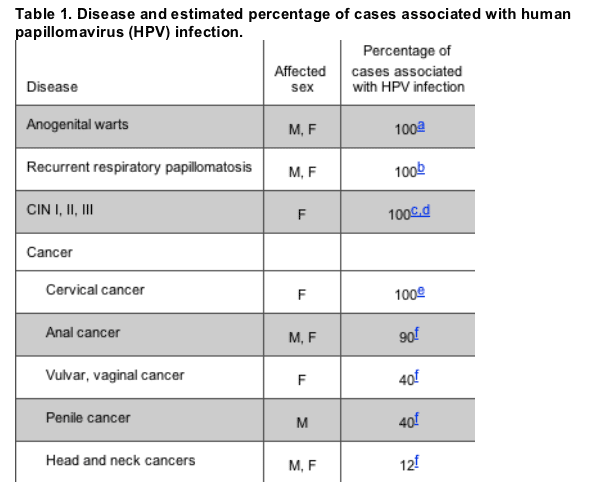
SYMPTOMATIC INFECTION
HPV infection is usually asymptomatic. Most infections are not associated with any signs or symptoms of infection, and many infections clear. HPV DNA testing is not recommended for inapparent or subclinical HPV infection, except in specific settings related to cervical cancer screening and management (see the Cervical Cancer Screening section below). In addition, HPV DNA testing is not recommended for persons with anogenital warts. There is no indication for HPV testing among men; infection in men is common, identifying infection does not prevent HPV-associated diseases or cancers, and no therapy has been identified that eradicates infection. In addition, examining male partners of women with subclinical infection (for whom Papanicolau test abnormalities have been found) has no known benefit. In this situation, most sex partners are likely to have already been infected.
CERVICAL CANCER
Cervical cancer is caused by high-risk HPV infection, although development of cancer often takes decades after initial infection [4]. The most common type of cervical cancer worldwide is squamous cell carcinoma, followed by adenocarcinoma. Both types of cervical cancer are caused by HPV; however, the contributing HPV types vary in different regions of the world. In the United States, there has been a substantial reduction in cervical cancer cases since the 1950s, when cervical cancer screening programs became standard. However, cervical cancer is still very common worldwide: it is the second most common cancer among women in the developing world. In most parts of the developing world, there are no cervical cancer screening programs that can detect and treat cervical cancer precursors and cancers at an early stage.
Data on the prevalence of cervical cancer in the United States are available from registries (National Cancer Institute Surveillance, Epidemiology and End Results program and the Centers for Disease Control and Prevention [CDC]-supported state cancer registries). Cervical cancer estimates are also made by the American Cancer Society (ACS) each year. The incidence of invasive cervical cancer was 8.7 cases per 100,000 women in the United States in 2002, and the incidence of cervical cancer death was 2.5 deaths per 100,000 women [30]. Estimates of the mortality rate for cervical cancer are 11.2 deaths per 100,000 patients in less-developed regions of the world [31].
CERVICAL CANCER SCREENING
Regular cervical cancer screening (through Papanicolau testing) and appropriate follow-up after abnormal test findings can prevent cervical cancer; however, the effectiveness of these programs depend on multiple screening visits and sometimes costly follow-up and treatment. In the United States, HPV infection is one of the most costly STIs, primarily because of the cost of screening, follow-up, and treatment that is associated with abnormal Papanicolau test findings [32].
Various professional organizations have recently revised their cervical cancer screening recommendations because of new data on the natural history and epidemiology of HPV infection, and because of results from clinical trials. Organizations such as the ACS, the American College of Obstetrics and Gynecology, and the United States Preventive Services Task Force now recommend that cervical cancer screening begin at age 21 years, or within 3 years of first sexual activity. Some organizations (such as the American College of Obstetrics and Gynecology and the ACS) also recommend specific uses of new technologies, including the HPV DNA test and liquid-based cytology. The high-risk HPV DNA test is recommended for use in the context of atypical squamous cells of undetermined significance (ASC-US) findings on Papanicolau test or among women receiving cervical cancer screening with a Papanicolau test who are 30 years of age [33-37].
ANOGENITAL WARTS
More than 90% of cases of anogenital warts are associated with the low-risk HPV types 6 and 11 [24]. Because anogenital warts are not routinely reported in the United States, national incidence is unknown. Population estimates, based primarily on STD clinic data, suggest that about 1% of the sexually active adolescent and adult populations in the United States have clinically apparent genital warts [38]. The average time to development of new anogenital warts following infection with HPV types 6 or 11 is relatively short (about 2-3 months) [39]; however, not all persons infected with HPV types 6 or 11 develop genital warts, and many of the risk factors for disease are incompletely described. One important risk factor for the development of anogenital warts is immunosuppression (HIV infection, receipt of an organ transplant, or renal disease). One study demonstrated that anogenital warts were highly infectious to sexual partners [40].
Anogenital warts can be treated; however, many warts regress without intervention. Treatment of anogenital warts is determined by wart size, location, number, patient preference, treatment cost, convenience, adverse effects, and provider experience [41]. Treatments are characterized as patient-applied or provider-applied modalities. Clearance of anogenital warts, whether associated with treatment or not, is commonly associated with recurrences (about 30%). Anogenital warts in HIV-infected individuals are often larger and more difficult to treat. Specific treatment options are available in the CDC STD treatment guidelines [41].
RECURRENT RESPIRATORY PAPILLOMATOSIS
Very rarely, HPV infection with low-risk HPV types, primarily types 6 or 11, causes respiratory tract warts or recurrent respiratory papillomatosis [42, 43]. A juvenile and adult form have been described; however, there are more data available on the juvenile form, also known as juvenile-onset recurrent respiratory papillomatosis. Juvenile-onset recurrent respiratory papillomatosis is believed to result from HPV transmitted from mother to infant during delivery. Estimates of the incidence rate of juvenile-onset recurrent respiratory papillomatosis are relatively imprecise, but according to a study performed in 2 cities in the United States, the incidence ranges from 0.12 to 2.1 cases per 100,000 children <18 years [42]. This disease can be debilitating for the child and can result in recurrent treatments-an average of 13 surgeries-to remove warts [43]. The prevalence and incidence of the adult form of recurrent respiratory papillomatosis is less clear.
ANOGENITAL CANCERS OTHER THAN CERVICAL CANCER
HPV is associated with other squamous cell intraepithelial neoplasias, carcinoma in situ, and anogenital cancers (penile, vaginal, vulvar, and anal). In the United States, these anogenital cancers are much less common than cervical cancer in women.
The age-adjusted incidence rates in 2002 were 1.2 cases per 100,000 men for anal cancer and 0.8 cases per 100,000 men for penile cancer. Among women, the age-adjusted incidence rates in 2002 were 1.5 cases per 100,000 for anal cancer, 2.3 cases per 100,000 for vulvar cancer, and 0.7 cases per 100,000 for vaginal cancer [30].
The incidence of anal cancer among men who have sex with men-in particular, those who are HIV infected-is higher than in the general population. The estimates based on data reported from urban areas suggested that men who have sex with men have a 17-fold increased risk of anal cancer [44]. Because of the increased incidence of anal cancer in certain populations, cytologic screening for anal cancers is recommended by some specialists. However, there are limited data on the natural history of anal intraepithelial neoplasias, the reliability of screening methods, the safety and response to treatments, and the programmatic considerations to support this screening approach. Such a screening approach may be beneficial, but more data are needed; at this time, the CDC does not recommend this screening approach [41].
HPV VACCINE
HPV vaccines are characterized as prophylactic or therapeutic. Therapeutic vaccinations are in very early stages of development and have shown limited success; however, 2 prophylactic HPV vaccines are in, or have completed, phase III clinical trials. These prophylactic vaccines are produced using recombinant DNA technology, in which capsid proteins (L1) for each HPV type are produced in cell culture. A unique attribute of the L1 capsid proteins is their ability to self-assemble into conformational VLPs. These proteins mimic the HPV virus, but they do not contain viral DNA.
VLPs to specific types are purified and used in combination with adjuvants for vaccines. One vaccine, produced by Merck, is a quadrivalent vaccine with HPV-6, -11, -16, and -18 VLPs. The other candidate vaccine, produced by GlaxoSmithKline, is a bivalent vaccine with HPV-16 and -18 VLPs. Both vaccines are administered in a 3-dose regimen. In June, 2006, the Merck quadrivalent HPV vaccine was licensed by the US Food and Drug Administration for use among female subjects aged 9-26 years. GlaxoSmithKline will likely submit a license application to the US Food and Drug Administration in late 2006. Both companies have applied for licensure in other countries throughout the world.
Evaluations of the bivalent and quadrivalent vaccines have demonstrated that vaccinated individuals develop high antibody titers to the respective HPV types [45-49]. These antibody titers are much higher than titers associated with natural infection. Both vaccines have also been shown to have high efficacy among female subjects who have not been exposed to the HPV vaccine types (table 2). Recently published results from a clinical trial evaluating women aged 16-23 years who received a monovalent HPV-16 vaccine demonstrated 100% efficacy for persistent HPV infection and 100% efficacy for outcomes of interest, such as cervical intraepithelial neoplasias I, II, or III, specific to HPV-16 [47]. In addition, data demonstrated that the Merck quadrivalent HPV vaccine has high efficacy for prevention of external genital lesions caused by vaccine types [48]. The Merck quadrivalent HPV vaccine demonstrated an overall 90% reduction in incident or persistent infection or genital disease associated with HPV types 6, 11, 16, and 18 [48]. Published results from clinical trials of the GlaxoSmithKline bivalent vaccine have demonstrated similar high efficacy [45, 49]. Results to date for the bivalent vaccine have found sustained efficacy up to 4.5 years; more than 98% seropositivity to HPV-16 and -18 VLPs was maintained, efficacy for incident infection was 97%, and efficacy for HPV persistence was 100% [49]. Both vaccines have been associated with minimal adverse reactions, the most common of which being local reactions. It is important to note that neither vaccine has been found to have efficacy against existing disease or infection due to HPV.
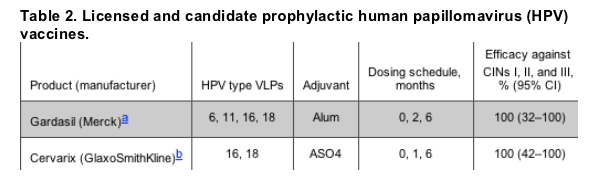
NOTE. Analyzed according to protocol, follow-up 36 months (Merck), and 54 months, combined initial and follow-up phase (GlaxoSmithKline). Alum, aluminum hydroxyphosphate sulfate; ASO4, aluminum hydroxide and monophosphoryl lipid A; CIN, cervical intraepithelial neoplasia; NA, not available; VLP, virus-like particle.
a Data are from [48]; Gardasil licensed for use by US Food and Drug Administration in 2006.
b Data are from [49].
HPV vaccines may have a significant impact on the prevalence and incidence of HPV infection, genital warts, cervical cancer precursor lesions, and HPV-associated cancers. A variety of models have been developed to evaluate the long-term impact and cost-effectiveness of an HPV vaccine; these models have demonstrated that HPV vaccines would be cost-effective when given to girls/women in the setting of current cervical cancer screening recommendations in the United States [50, 51]. Estimates of cost-effectiveness vary and depend on parameters such as screening frequency, duration of protection, coverage of the vaccine, and age of patient at vaccination.
The HPV vaccine appears to be acceptable to parents and providers. A survey of pediatricians in 2004 suggested that providers are interested in administering this vaccine to adolescents. In this study, there was higher intent to vaccinate older, compared with younger, adolescents [52]. Consistent themes in provider evaluations have been the importance of professional organizations and a reluctance to vaccinate younger adolescents [52, 53]. Parental acceptability surveys have raised questions about HPV knowledge and willingness to accept administration to adolescents of a vaccine for an STI. It is important to note, however, that the majority of parents surveyed recognize the benefits of an HPV vaccine [53].
The Advisory Committee on Immunization Practices made recommendations for the use of the quadrivalent vaccine in June 2006. These provisional recommendations include routine vaccination of girls 11-12 years of age; vaccine may be given to girls as young as 9 years of age. In addition, vaccination of girls and women aged 13-26 years (who have not received or completed the vaccine series) is recommended. Optimally, the vaccine would be given to young adolescents, a group that is unlikely to have had sexual intercourse. In the United States, 25% of girls have had sexual intercourse by age 15 years [54]. As described earlier, because HPV acquisition occurs soon after sexual initiation, the vaccine would have greater effectiveness if administered to young adolescents (age, 11-12 years) before they have acquired vaccine HPV types. In addition, young adolescents are likely to be receiving other vaccines, including vaccines for meningococcal infection and tetanus/diphtheria/pertussis; thus, implementation of vaccination may be easier for this age group than for older adolescents.
Questions remain about the impact and cost-effectiveness of preventing HPV-associated conditions by vaccinating women and men, or by vaccinating women only. The immunogenicity and safety of the quadrivalent vaccine has been similar in young men and women. Although there are potential benefits to vaccinating men, to date, there are efficacy data only for women. The efficacy of an HPV vaccine for anogenital warts and cancers in men is unknown. Further clinical studies will evaluate efficacy in this group. In addition, modeling efforts are underway to evaluate the impact of vaccinating men on outcomes among both men and women.
Adolescent vaccination may present challenges for implementation, including access to adolescents and delivery and completion of a 3-dose schedule. Evaluations to improve vaccine uptake may be especially important for this vaccine. There are additional unanswered questions about HPV vaccination that have public health importance, including longevity of protection, the effect of the vaccine on other nonvaccine HPV types, and how this vaccine will impact existing cervical cancer screening practices. Routine cervical cancer screening will continue to be necessary, because there are additional HPV types (beyond those addressed by the vaccines) that lead to cervical cancer precursors and cancer.
There are promising prevention strategies and uses for new technologies for cervical cancer screening. The integration of these new strategies and technologies into existing cervical cancer programs may result in decreased morbidity and mortality related to HPV infection in the United States. These strategies may provide even greater benefit in the areas of the world in where there is higher morbidity and mortality related to HPV infection.
|
|
| |
| |
|
|
|