| |
Longitudinal Analysis of Bone Density in HIV-Infected Women
|
| |
| |
The Journal of Clinical Endocrinology & Metabolism Aug 2006 91(8):2938-2945
Sara E. Dolan, Jenna R. Kanter, and Steven Grinspoon
Program in Nutritional Metabolism (S.E.D., J.R.K., S.G.), Massachusetts General Hospital, and Harvard Medical School (S.G.), Boston, Massachusetts 02114
ABSTRACT
Objectives: The objective of the study was to investigate change in bone mineral density (BMD) over time in HIV-infected women in comparison with healthy control subjects similar in age, race, and body mass index (BMI).
Design: This was a prospective cohort study.
Methods: BMD was measured by dual-energy x-ray absorptiometry in 100 HIV-infected females and 100 healthy controls similar in age (41 ± 1 vs. 41 ± 1 yr,
P=0.57), BMI (26.1 ± 0.5 vs. 27.2 ± 0.4 kg/m2, P =0.12), and race (60 vs. 65% non-Caucasian, P 0.47, HIV-infected vs. controls). Changes in BMD were determined every 6 months over 24 months.
Results:
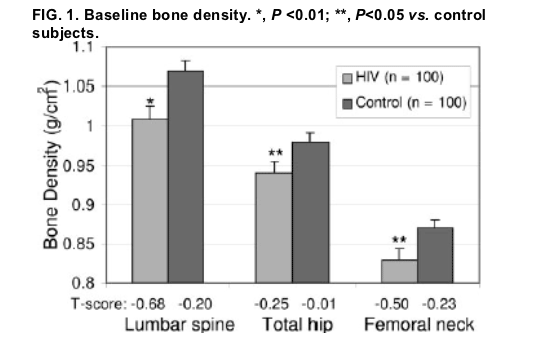
At baseline, HIV-infected subjects had lower BMD at the lumbar spine (1.01± 0.01 vs. 1.07± 0.01 g/cm2, P =0.001), hip (0.94 ±0.01 vs. 0.98± 0.01 g/cm2, P =0.02), and femoral neck (0.83 ± 0.01 vs. 0.87 ± 0.01 g/cm2, P =0.02).
Historical low weight, duration of nucleoside reverse transcriptase inhibitor use, and FSH were significantly associated with lumbar BMD, whereas duration of HIV, BMI, historical low weight, smoking pack-years, N-telopeptide of type 1
collagen, viral load, 25 hydroxyvitamin D, and osteocalcin were associated with hip BMD at baseline.
In mixed model longitudinal analyses, BMD remained lower in HIV-infected subjects than in controls over 24 months of follow-up (P =0.001 for the spine, P=0.04 for the hip, and P =0.02 for the femoral neck). These differences remained significant controlling for age, race, BMI, and menstrual
function.
In contrast, rates of change for the spine (P=0.79), hip (P =0.44), and femoral neck (P=0.34) were not different between the HIV and control groups over 2 yr.
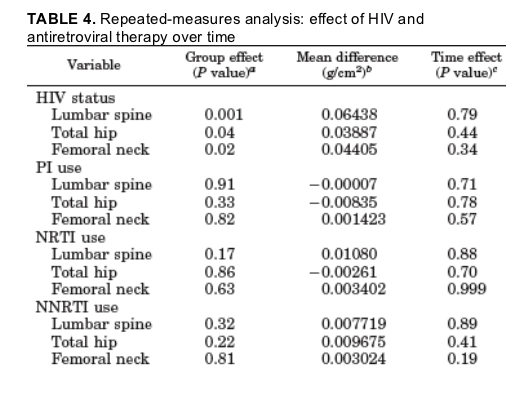
In the HIV group, longitudinal changes in BMD were not associated with current protease inhibitor, nucleoside reverse transcriptase inhibitor, or non-nucleoside reverse transcriptase inhibitor use but were associated with CD4 count, weight, FSH, N-telopeptide of type 1 collagen, and baseline BMD.
Conclusions:
BMD is reduced at the spine, hip, and femoral neck among women with HIV in relationship to low weight, duration of HIV, smoking, and increased bone turnover. Over 2 yr of follow-up, BMD remained stable but lower in HIV-infected women, compared with control subjects.
Background
REDUCED BONE DENSITY is seen among HIV-infected men (1- 6). In initial studies, protease inhibitor (PI) use was thought to be associated with bone loss, but subsequent studies have not consistently confirmed an effect of PIs or other antiretroviral agents on bone mineral density (BMD) (2, 7-9). In contrast, newer studies suggest that traditional risk factors such as weight and increased bone turnover may contribute to increased bone loss (5, 6, 10). Despite the growing
rate of HIV infection among women in the United States (11), a limited number of studies have examined bone density among female patients with HIV disease. Prior cross-sectional studies have shown reduced bone density in HIV-infected
women with wasting syndrome and among women with stable weight (4, 5) as well as among postmenopausal HIV-infected women (12). However, changes in bone density over time have not been assessed in HIV-infected women. In
this prospective cohort study, we perform longitudinal assessment of bone density among women with HIV and healthy weight-, age-, and race-matched control subjects. We further assess the effects of traditional risk factors and antiretroviral therapy on BMD.
Discussion
In the present study, we demonstrate that bone density of the lumbar spine, total hip, and femoral neck is reduced in HIV-infected women. These data extend the findings of our prior cross-sectional study (5) to show the pattern of bone density over 2 yr of follow-up. An important finding in this regard is that bone density is reduced but stable over 2 yr of follow-up, arguing against ongoing active bone loss relative to a well-matched control population. These data provide
reassurance that bone loss is moderate and not progressive, at least among relatively young, weight-stable, HIV-infected patients with good virologic control.
We could find no evidence in longitudinal modeling of any effects of current PI, NRTI, or NNRTI on bone density. At baseline, bone density was reduced at the hip, femoral neck, and spine among the HIV-infected patients, compared with a control population. However, bone loss was moderate, as shown by T scores. We again show that approximately 41% of HIV-infected women will have osteopenia at the hip, femoral neck, or spine, but few patients demonstrated osteoporosis (7%).
Our data highlight that bone turnover is increased among HIV-infected women and that the increased bone turnover is a factor contributing to low bone density along with traditional factors such as low weight and smoking. Differences in bone density are likely multifactorial, and independent factors contributing to bone loss may be difficult to determine in cross-sectional modeling.
In this study, we were also able to perform longitudinal analyses and modeling of factors contributing to bone loss among HIV-infected women. For example, increased baseline NTx (NTx, N-telopeptide of type 1 collagen) is associated with lower bone density at baseline and predicts greater rates of bone loss over time. The mechanisms of increased bone turnover among HIV-infected women are unclear and are not likely the result of estrogen deficiency because estradiol and gonadotropin levels were similar to the control group and subjects were largely premenopausal.
HIV itself may increase bone turnover, as reported by Aukrust et al. (17), and this may relate to increased cytokines such as TNF and IL-6, as we have previously
shown (5). Prior studies have implied that antiretroviral therapy may
contribute to reduced bone density among this population (7, 18, 19). In this study, we demonstrate that duration NRTI use may be associated with bone loss, especially in the spine in both cross-sectional and longitudinal modeling. In contrast, current use of NRTI therapy was not associated with bone loss. The association between duration of NRTI use and lumbar spine bone density was independent of HIV duration and other traditional risk factors. The mechanism by which NRTI use might contribute to bone loss is not clear. Chronic NRTI therapy may be associated with lactic acidemia, and prior investigations have shown a relationship between lactic acidemia and osteopenia in HIV-infected men (19). The HIV-infected women in the present study were found to have significantly elevated lactate levels. However, lactic acid levels were not related to bone, and the relationship with NRTI treatment was independent of lactate levels in regression modeling. It is also possible that NRTI use may be a marker for more severe initial disease. Moreover, specific NRTIs may affect bone differently, and our study was not designed to examine the effects of individual antiretroviral agents on bone. Importantly, our longitudinal data argue against a short-term effect of current NRTI treatment but suggest that long-term duration of treatment may be associated with bone effects.
Neither our cross-sectional nor longitudinal data suggest an effect of PI on bone. These data are in agreement with Nolan et al. (20), who did not find evidence of accelerated bone loss in patients with PI-containing highly active antiretroviral therapy regimens, and other investigators, who did not find a relationship between bone loss and type or duration of antiretroviral therapy use (2, 9, 21). Collectively, these findings are in contrast with the findings of Tebas et al. (7) and others (22), who found a relationship between osteoporosis and osteopenia in patients receiving PI therapy.
Further prospective studies are needed to determine whether PIs affect bone density. The HIV-infected patients that we studied had a mean age of 41 yr and were largely premenopausal, reflecting the demographic of the disease among women (23). Indeed, we do not show ongoing further loss in either the HIV-infected or control group over 2 yr of follow-up, consistent with the relatively young age of the patients studied.
As the HIV population ages, more women will become postmenopausal, and such patients may be at increased risk for bone loss if they had increased bone turnover due to HIV or other factors in the perimenopausal period. Yin et al. (12) recently demonstrated increased bone loss among postmenopausal HIV-infected women. Indeed, FSH was a significant predictor of
lower bone density at the lumbar spine among younger HIV-infected subjects in our study and was also significant as a predictor of longitudinal changes in BMD. Because 77% of our population were premenopausal by FSH levels, we did
not include enough postmenopausal women to perform a separate subanalysis in this group. However, we were able to show that our results remained similar in a subanalysis, strictly limited to premenopausal women. Further longitudinal
studies of bone density among postmenopausal women with HIV disease are critical to determine optimal testing and treatment paradigms.
Prior studies have suggested that 25-hydroxyvitamin D levels are lower among those with HIV (20, 24-26). We found a trend for lower 25-hydroxyvitamin D levels among our HIV-infected female subjects, but neither vitamin D nor calcium
intake was reduced, compared with control subjects. However, at least for the hip, lower 25-hydroxyvitamin D levels were significantly associated with reduced bone density. In contrast, baseline 25-hydroxyvitamin D levels were not significant in longitudinal modeling for bone density. Our data suggest that assessment of 25-hydroxyvitamin D levels may be important among HIV-infected women with bone loss, but further study is needed in this regard.
A limitation of our study is the high dropout rate associated with the longitudinal follow-up of HIV-infected women, who have many psychosocial and sociodemographic barriers to study participation. However, we performed
detailed validation analyses, demonstrating that rates of bone loss were not different among those dropping out and continuing on, e.g. the dropouts were at random and did not confound the results. Furthermore, our results remain significant, controlling for age, race, BMI, and menstrual function, suggesting differences due to HIV itself.
Our study provides novel longitudinal bone density data among HIV-infected women demonstrating relative stability of bone density over time in a largely premenopausal population with stable weight, even in the presence of ongoing
antiretroviral therapy. Our data cannot be generalized to older women or women with more severe HIV disease or ongoing weight loss, in whom bone density may be more severely reduced over time.
Our data suggest that initial bone density screening may be useful for HIV patients at risk for bone loss because of low weight, increased bone turnover, or high FSH levels. Women with significant bone loss and increased bone turnover may be candidates for bisphosphonate therapy, once other factors such as 25-hydroxyvitamin D deficiency have been corrected (27). Low initial bone density strongly predicted further change in bone density at all three sites, providing further rationale for screening and treatment in this population. However, our data suggest that younger women with minimal or mild loss can be expected to maintain stable bone density at least over the short term.
Results
Baseline demographic data
One hundred HIV-infected women and 100 HIV-negative control subjects completed the baseline visit. The HIV- infected and control subjects recruited for this study were similar in age, BMI, and race (Table 1). HIV-infected subjects
were less likely to be eumenorrheic (59 vs. 82%, P =0.0004, HIV infected vs. controls), but age of menarche (13 ± 0 vs. 13 ± 0 yr, P= 0.47) was not different between the groups. A greater percentage of HIV-infected patients were receiving hormone replacement therapy (7 vs. 1%, P =0.03, HIV infected
vs. controls). Fourteen percent of the HIV-infected women vs. 3% of the control subjects had a hysterectomy. A larger percentage of the HIV-infected women were current cigarette smokers (Table 1). Among those with HIV, 81% reported current use of antiretroviral medication, and the mean duration of HIV was
8.1 ± 0.4 yr. Immune function and medication data are shown in Table 1. The numbers of patients on individual antiretrovirals are shown in the legend for Table 1. The demographic data of the subset of 50 subjects who completed the 24-month study did not differ between groups (Table 1).
Baseline bone density and biochemical data
The HIV-infected subjects had significantly lower bone density at the lumbar spine (1.01 ± 0.01 vs. 1.07± 0.01 g/cm2, P =0.001), hip (0.94 ± 0.02 vs. 0.98 ± 0.01 g/cm2, P=0.02), and femoral neck (0.83± 0.01 vs. 0.87± 0.01 g/cm2, P=0.02), compared with the age-, race-, and weight-matched control subjects (Fig. 1). Similar results were obtained in a subset limited to premenopausal HIV (n=79) and control (n =89) subjects (lumbar spine, 1.03 ± 0.02 vs. 1.08 ±0.01 g/cm2, P=0.01; hip, 0.95 ± 0.02 vs. 0.99 ± 0.01 g/cm2, P=0.055; and femoral neck, 0.84 ± 0.01 vs. 0.88 ± 0.01 g/cm2, P=0.046, HIV infected vs. control subjects, respectively). In addition, similar results were obtained and significant differences remained between HIV and control groups controlling for
hysterectomy and HRT use (P =0.011, P=0.036, P =0.029 for lumbar spine, hip, and femoral neck, respectively). Forty-one percent of the HIV-infected women demonstrated osteopenia at the hip, femoral neck, or spine, and 7% demonstrated osteoporosis.
HIV-infected subjects had significantly higher osteocalcin (4.1 ± 0.3 vs. 3.0 ± 0.1 nm/liter, P=0.001) and urine NTx levels (40.9 ± 3.2 vs. 30.3 ± 1.8 nm/mm creatinine, P=0.007), compared with the control subjects (Table 2). Serum 25-
hydroxyvitamin D, calcium, and urine creatinine levels were not different between the groups. The HIV-infected subjects had significantly higher lactic acid levels (0.99 ± 0.06 vs. 0.70 ± 0.03 mmol/liter, P=0.0001) than the control subjects. Estradiol, LH, and FSH were not different between the groups. Based on FSH greater than 20 IU/liter, 23 and 12% of HIV and control subjects, respectively, were menopausal.
Baseline body composition and dietary measures
The HIV-infected subjects had significantly lower total fat mass, compared with the control subjects (22.7 ± 0.9 vs. 26.1 ± 0.9 kg, P=0.009), whereas lean body mass was not significantly different between the groups (Table 2). The
HIV-infected subjects reported a higher daily consumption of vitamin D (10.5 ± 1.8 vs. 6.9 ± 0.6 g, P=0.055), although no difference was seen in daily intake of calcium, protein, fat, or total calories between the two groups.
Baseline regression modeling
A stepwise regression analysis with fit model testing for baseline lumbar spine, total hip, and femoral neck bone density was performed among the HIV-infected subjects. Overall R2 were 0.44, 0.52, and 0.36 for the spine, hip, and neck
models, respectively (Table 3). Historical low weight (beta=0.0016918 g/cm2 per kg increase, P =0.0006), duration of NRTI use (beta=0.01074 g/cm2 per yr, P=0.007), and FSH (beta = -0.001954 g/cm2 per IU/liter increase, P<0.0001)
were associated with lumbar BMD, whereas duration of HIV (beta= -0.007524 g/cm2 per yr, P 0.03), BMI (beta= 0.0068366 g/cm2 per kg/m2 increase, P=0.046), historical low weight (beta=0.0022854 g/cm2 per kg increase,
P=0.0003), smoking pack-years (beta= -0.002303 g/cm2 per pack-year, P=0.04), NTx (beta= -0.00136 g/cm2 per nm NTx/mm creatinine increase, P=0.04), viral load (beta=0.0025 g/cm2 per 1000 copies/ml increase, P=0.003), 25-
hyroxyvitamin D (beta=0.0055081 g/cm2 per nmol/liter increase, P=0.008), and osteocalcin (beta=0.0030517 g/cm2 per nmol/liter increase, P=0.0003) were associated with hip BMD, and historical low weight (beta=0.0020432 g/cm2 per kg increase, P =0.0005), smoking pack-years (beta= -0.002955 g/cm2 per pack-year, P 0.02), and osteocalcin (beta= 0.0019253 g/cm2 per nmol/liter increase, P=0.03) were associated with femoral neck BMD (Table 3).
Repeated-measures longitudinal analysis
In mixed-model longitudinal analyses, BMD in the HIV-infected subjects remained lower than in control subjects over 24 months of follow-up (P=0.001 for the spine, P=0.04 for the hip, and P=0.02 for the femoral neck) (Table 4 and Fig. 2) in unadjusted analyses.
HIV status remained significant in the longitudinal analysis of lumbar spine and femoral neck bone density, controlling for age, race, BMI, and menstrual function as covariates. HIV status also remained significant in the longitudinal analysis of hip bone density controlling for age, race, and menstrual function and approached significance (P=0.07) controlling for BMI. Age and BMI were significant in all models, whereas race was significant for the hip and femoral neck but not the lumbar spine. Menstrual function was not significant in any of the models. In contrast, rates of change for the spine (P =0.79), hip (P=0.44), and femoral neck (P=0.34) were not different between the HIV and control groups over the 2-yr follow-up period (Table 4 and Fig. 2).
A validation analysis was performed, demonstrating that the change in bone density over 6 months was not different among HIV infected patients dropping out after 6 months vs. those who continued beyond 6 months (P =0.34, P =0.63,
and P =0.15 for HIV at the spine, hip, and femoral neck, respectively), and similarly no differences were seen in the same analysis for control subjects (P=0.92, P=0.72, and P =0.39 for controls at the spine, hip, and femoral neck, respectively) (Table 5). Similar analyses were performed for the 12- and 18-month time periods without significant differences.
None of the patients were on bisphosphonates at baseline, and similar numbers (two patients in the HIV group and one in the control group) were using such treatment by month 24 of the study. Similar numbers of patients, one in the HIV
group and two in the control group, were receiving estrogen at month 24 of the study.
Among the HIV patients, longitudinal changes in BMD were not seen in relationship to current PI, NRTI, or NNRTI use in unadjusted or adjusted models (Table 4). In modeling for all three drug classes (PI, NRTI, and NNRTI), CD4, BMI, lowest adult weight, and FSH were significant predictors of change in bone density over time at the lumbar spine, hip, and femoral neck (P at all sites in modeling for all three drug classes. In contrast, viral load, smoking, 25-hydroxyvitamin D, and osteocalcin were not significant predictors of change in BMD at any site in any of the models. NTx was a significant predictor of change in femoral neck BMD in modeling for PI and NNRTI effects and for
lumbar spine in modeling for NNRTI effects. Duration of antiretroviral therapy use was a significant predictor of change in lumbar bone density in modeling for NRTI use but not in other models. All available data were included in the repeated-measures analysis (see Fig. 2 for number of subjects with available data at each time point). A total of 25 HIV-positive subjects and 25 control subjects completed the 24-month study visit. A repeated-measures analysis was also performed for weight, using BMI at each time point. Weight did not change between the study groups at each time point over 24 months (P= 0.77).
Subjects and Methods
Subjects
One hundred HIV-infected women and 100 HIV-negative female controls were recruited through community advertisement and primary care provider referral between March 2000 and May 2004. Subjects who used megace, ketoconazole, antidiabetic agents, bisphosphonates, steroids, GH, oral contraception pills, Depo Provera, Progestasert intrauterine device, testosterone, or any other anabolic agents within the prior 3 months were excluded. Subjects who engaged in substance abuse, were pregnant or breast-feeding in the past year, had a history of oophorectomy, or were diagnosed with an illness affecting bone were
also excluded from participation. Inclusion criteria for HIV-infected participants included age between 18 and 60 yr, previously diagnosed HIV infection, stable antiretroviral regimen, and a body mass index (BMI) between 20 and 35 kg/m2. Duration of HIV and antiretroviral medication history was obtained via patient interview at the screen visit, as was data regarding menstrual history and age of menarche. Menstruating women were characterized as eumenorrheic (normal menstrual function), oligomenorrheic (less than three menstrual periods in the 3 months before study), or amenorrheic (complete absence of period 3 months before study). Female control subjects met the same entrance criteria, and ELISA testing verified HIV-negative status. All subjects were encouraged to return for a visit identical with the baseline visit every 6 months for a total of 24 months. Bone density data were made available equally for subjects and controls.
Bone density assessment
BMD of the lumbar spine and hip (total hip and femoral neck) was measured by dual x-ray absorptiometry (DXA) using a Hologic 4500 densitometer (Hologic Inc., Waltham, MA). The in vivo precision for the measurement of bone density using theDXAtechnique is 0.5-1.5% at the lumbar spine (13), and the sd of the lumbar spine bone density is 0.01 g/cm2 (14). Osteopenia and osteoporosis were defined according to World Health Organization criteria (15) (osteopenia: T score < -1.0 sd and >/= 2.5 sd; osteoporosis: T score < - 2.5 sd). Ethnicity-specific T
scores provided by the manufacturer were used to determine osteopenia and osteoporosis (Hologic).
Body composition measurement
BMI was calculated from fasting weight and height measurements. Total fat and lean body mass were measured by DXA. The DXA technique has a precision error of 3.0% for total body fat mass and 1.5% for total lean mass (16).
Nutrition evaluation
Participants completed a 4-d food record before their visit. The food records were reviewed with each patient by a registered dietitian and analyzed using a computerized nutrition software product (NDS versions 4.01 and 4.02-NDS-R, Regents of the University of Minnesota, Minneapolis, MN) to quantify total protein, fat, and caloric intake as well as total calcium and vitamin D intake (including supplements). Historic low adult body weight was determined by patient interview.
|
|
| |
| |
|
|
|