| |
Fish Reduces Risk of Dementia by 47% in Framingham Heart Study
|
| |
| |
"Plasma Phosphatidylcholine Docosahexaenoic Acid Content and Risk of Dementia and Alzheimer Disease"
The Framingham Heart Study
Ernst J. Schaefer, MD; Vanina Bongard, MD, PhD; Alexa S. Beiser, PhD; Stefania Lamon-Fava, MD, PhD; Sander J. Robins, MD; Rhoda Au, PhD; Katherine L. Tucker, PhD; David J. Kyle, PhD; Peter W. F. Wilson, MD; Philip A. Wolf, MD
Lipid Metabolism Laboratory (Drs Schaefer, Bongard, and Lamon-Fava) and Dietary Assessment and Epidemiology Program (Dr Tucker), Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, Department of Biostatistics, Boston University School of Public Health (Dr Beiser), and Department of Endocrinology, Nutrition, and Diabetes (Dr Robins) and Department of Neurology (Drs Au and Wolf), Boston University School of Medicine, Boston, Mass; Martek Biosciences Corporation, Columbia, Md (Dr Kyle); and Framingham Heart Study, National Heart, Lung, and Blood Institute, Framingham, Mass (Drs Wilson and Wolf).
Arch Neurol. Nov 11, 2006;63:1545-1550.
INTRODUCTION
Dementia is a major cause of disability among the elderly, with Alzheimer disease being responsible for about 70% of cases. Age, family history, and the presence of the apolipoprotein E 4 allele have been found to be significant risk factors for the development of Alzheimer disease and all-cause dementia.1-3 More recently, a high plasma concentration of homocysteine has also been shown to be a risk factor for Alzheimer disease and dementia.4
Docosahexaenoic acid (DHA), an -3 polyunsaturated fatty acid found in some foods and many tissues in the body, also appears to be important in affecting the risk of dementia. Docosahexaenoic acid can be formed from -linolenic acid, an essential fatty acid that must be obtained from the diet or can be obtained directly by consuming foods rich in DHA such as fish or fish oil or supplements containing DHA. Docosahexaenoic acid appears to be important for central nervous system function.5-6 Cross-sectional studies have linked low DHA levels with dementia,7-10 while prospective studies have linked all-cause dementia and Alzheimer disease with decreased fish intake.11-14 Our hypotheses in this study were that plasma phosphatidylcholine (PC) DHA content is related to the risk of all-cause dementia and Alzheimer disease, as well as to dietary DHA and fish intake.
"....In this cohort free of dementia at baseline, plasma PC DHA (phosphatidylcholine docosahexaenoic acid) content predicted the occurrence of new dementia, independent of age, sex, apolipoprotein E 4 genotype, plasma homocysteine concentration, and education level. Subjects with baseline plasma PC DHA levels in the upper quartile experienced a significant 47% lower risk of dementia compared with participants with levels in the lower 3 quartiles. No other plasma PC fatty acid was independently linked to the risk of dementia.... a 50% reduction in the risk of Alzheimer disease was associated with the consumption of more than 2 servings of fish per week.... Plasma PC DHA content is determined by the degree of conversion of a-linolenic acid to DHA within the liver and by the consumption of foods rich in DHA. In our study, the correlation between plasma PC DHA content and fish intake was significant, indicating that fish intake is an important source of dietary DHA. Furthermore, subjects with plasma PC DHA levels in the highest quartile were those with the greatest fish consumption..."
ABSTRACT
Background Docosahexaenoic acid (DHA) is an abundant fatty acid in the brain. In the diet, DHA is found mostly in fatty fish. The content of DHA has been shown to be decreased in the brain and plasma of patients with dementia.
Objective To determine whether plasma phosphatidylcholine (PC) DHA content is associated with the risk of developing dementia.
Design, Setting, and Participants A prospective follow-up study in 899 men and women who were free of dementia at baseline, had a median age of 76.0 years, and were followed up for a mean of 9.1 years for the development of all-cause dementia and Alzheimer disease. (Framingham study)
Main Outcome Measures Plasma PC fatty acid levels were measured at baseline. Cox proportional regression analysis was used to assess relative risks of all-cause dementia and Alzheimer disease according to baseline plasma levels.
Results
Ninety-nine new cases of dementia (including 71 of Alzheimer disease) occurred during the follow-up. After adjustment for age, sex, apolipoprotein E 4 allele, plasma homocysteine concentration, and education level, subjects in the upper quartile of baseline plasma PC DHA levels, compared with subjects in the lower 3 quartiles, had a relative risk of 0.53 of developing all-cause dementia (95% confidence interval, 0.29-0.97; P=.04) and 0.61 of developing Alzheimer disease (95% confidence interval, 0.31-1.18; P=.14). Subjects in the upper quartile of plasma PC DHA levels had a mean DHA intake of 0.18 g/d and a mean fish intake of 3.0 servings per week (P<.001) in a subset of 488 participants. We found no other significant associations.
[....In an editorial in the same issue, Martha Morris, Sc.D., of Rush y in Chicago wrote that the Schaefer trial provides the first evidence that direct measurement of DHA in plasma is related to lower Alzheimer's risk....She wrote that on the basis of available epidemiological evidence, the amount of DHA required for a protective benefit would be one fish meal a week, 0.18g/d of DHA, (based on one 3-oz serving of cooked Atlantic salmon, for example)....] note from Jules Levin: I think other factors besides fish intake may effect DHA levels so for some individuals more than 1 fish meal a week may be required.
Conclusion The top quartile of plasma PC DHA level was associated with a significant 47% reduction in the risk of developing all-cause dementia in the Framingham Heart Study.
"...To further investigate the relationship between DHA and risk for dementia, analyses of dietary DHA and fish intake were performed in the subsample of 488 participants who completed the semiquantitative food frequency questionnaire at the beginning of the follow-up period, as shown in Table 4. Mean DHA intake and fish intake were significantly (both P<.001) associated with plasma PC DHA levels by quartile. Adjusted mean (SE) fish intake ranged from 1.3 (0.2) to 3.0 (0.2) servings per week....
....Eating fish more than twice a week compared to at most 2 servings reduced risk by 39%: The adjusted RRs in subjects consuming fish more than twice a week compared with those consuming, at most, 2 servings of fish per week were 0.61 (95% CI, 0.28-1.33; P=.22) for all-cause dementia and 0.50 (95% CI, 0.20-1.27; P=.14) for Alzheimer disease..."
RESULTS
The cohort was followed up for a mean period of 9.1 years. Ninety-nine subjects developed dementia during the follow-up period (including 71 cases of Alzheimer disease). The baseline characteristics of the sample (at the 20th biennial examination) are shown in Table 1. Mean (SD) plasma PC DHA level was equal to 3.5% (1.1%) of total fatty acids in men, 3.7% (1.1%) in women, and 3.6% (1.1%) for the whole population. The top quartile had values of greater than 4.2%.
Results of the Cox proportional hazards regression analyses assessing the RRs of all-cause dementia associated with baseline plasma PC DHA levels are shown in Table 2. The adjusted RR of all-cause dementia per increment of 1 SD in the log-transformed baseline plasma PC DHA value was 0.80 (95% confidence interval [CI], 0.65-1.00; P=.047). We found no significant difference in risk between subjects in quartile 1 vs those in quartile 2 or 3. After adjustment for age and sex, subjects in the highest quartile had an RR of 0.53 (95% CI, 0.29-0.98; P=.04), compared with those in quartile 1. The RR decreased to 0.52 (95% CI, 0.26-1.04; P=.07) after further adjustments for the 4 allele of apolipoprotein E, homocysteine concentration, and education level. When subjects with baseline plasma PC DHA levels in the upper quartile were compared with the subjects with levels in all lower 3 quartiles, the RR was 0.53 (95% CI, 0.29-0.97; P=.04) after adjustment. These data indicate a substantial reduction in risk for all-cause dementia in the highest quartile group.
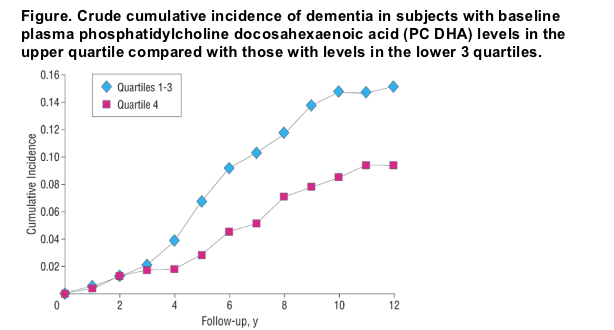
The Figure shows the crude cumulative incidence of dementia among subjects in the upper plasma PC DHA quartile and among subjects in the lower 3 quartiles. In Table 3, the results for all-cause dementia are compared with the results for the development of Alzheimer disease. The RR of developing Alzheimer disease varied from 0.59 to 0.61 when the top quartile was compared with the other 3 quartiles, depending on which variables were adjusted for. None of these differences in RR reached statistical significance, although the trends were similar to those observed for all-cause dementia. Further adjustments for body mass index, hypertension, diabetes mellitus, smoking status, alcohol intake, and history of stroke did not appreciably change these results (RR for all-cause dementia, 0.54 [95% CI, 0.29-0.98; P=.04]; RR for Alzheimer disease, 0.62 [95% CI, 0.32-1.22; P=.17]). No adjustments were made for total or high-density lipoprotein cholesterol levels because no significant associations were noted with the risk of all-cause dementia or, as recently reported from the Framingham Heart Study, with the risk of Alzheimer disease.24
Similar analyses were performed to assess the RRs of dementia and Alzheimer disease associated with plasma PC levels of -linolenic acid, eicosapentaenoic acid, linoleic acid, arachidonic acid, oleic acid, palmitic acid, and stearic acid. After adjustment for age and sex, none was significant except the RR of Alzheimer disease associated with a 1-SD increase in the log-transformed level of plasma PC linoleic acid (RR, 1.27 [95% CI, 1.01-1.61; P=.045]). However, this RR did not remain significant after further adjustment for the presence of the apolipoprotein E 4 allele, homocysteine concentration, and education level (RR, 1.24 [95% CI, 0.97-1.59; P=.09]).
To further investigate the relationship between DHA and risk for dementia, analyses of dietary DHA and fish intake were performed in the subsample of 488 participants who completed the semiquantitative food frequency questionnaire at the beginning of the follow-up period, as shown in Table 4. Mean DHA intake and fish intake were significantly (both P<.001) associated with plasma PC DHA levels by quartile. Adjusted mean (SE) fish intake ranged from 1.3 (0.2) to 3.0 (0.2) servings per week.
The RRs of all-cause dementia and Alzheimer disease associated with dietary DHA and fish intakes were studied by Cox proportional hazards regression analysis. After adjustment, the RR for all-cause dementia in subjects with dietary DHA intake in the upper quartile compared with those with intake in the lower 3 quartiles was 0.56 (95% CI, 0.23-1.40; P=.22). The RR of developing Alzheimer disease after adjustment in this same group was 0.63 (95% CI, 0.23-1.72; P=.37). The adjusted RRs in subjects consuming fish more than twice a week compared with those consuming, at most, 2 servings of fish per week were 0.61 (95% CI, 0.28-1.33; P=.22) for all-cause dementia and 0.50 (95% CI, 0.20-1.27; P=.14) for Alzheimer disease.
COMMENT
In this cohort free of dementia at baseline, plasma PC DHA content predicted the occurrence of new dementia, independent of age, sex, apolipoprotein E 4 genotype, plasma homocysteine concentration, and education level. Subjects with baseline plasma PC DHA levels in the upper quartile experienced a significant 47% lower risk of dementia compared with participants with levels in the lower 3 quartiles. No other plasma PC fatty acid was independently linked to the risk of dementia.
Plasma PC DHA content is determined by the degree of conversion of a-linolenic acid to DHA within the liver and by the consumption of foods rich in DHA. In our study, the correlation between plasma PC DHA content and fish intake was significant, indicating that fish intake is an important source of dietary DHA. Furthermore, subjects with plasma PC DHA levels in the highest quartile were those with the greatest fish consumption. However, fish intake accounted for less than half of the variability in DHA levels. The major fatty acids in fish are DHA and eicosapentaenoic acid. We found no relationship of dementia with plasma PC eicosapentaenoic acid level, whereas the association with plasma PC DHA level was significant. This is consistent with earlier data showing high levels of DHA in brain tissue,5-6 and the report of low DHA content in the brain of individuals with Alzheimer disease.7
A number of investigators8-9 have previously analyzed the links between plasma DHA status and dementia and documented lower plasma phospholipid or cholesteryl ester DHA content in patients with dementia or impaired cognitive function compared with healthy elderly subjects. An inverse association between cognitive decline and the ratio of w-3 to w-6 fatty acids in erythrocytes has been reported as well.10 Furthermore, increased fish and DHA intake were also found to be protective against cognitive decline and the risk of developing Alzheimer disease.11-14 Similarly, in our study, a 50% reduction in the risk of Alzheimer disease was associated with the consumption of more than 2 servings of fish per week.
To our knowledge, our study is the first prospective analysis to assess the predictive value of plasma PC DHA content in the occurrence of dementia and Alzheimer disease. Its strengths lie in its prospective design, its long follow-up period (9 years on average), the size of the sample, and the analysis of dietary data along with the direct assessment of the association between dementia and plasma phospholipid fatty acid content. Its limitations are that plasma PC DHA levels were measured on only 1 occasion, that dietary data were available for only a subset of nonrandomly selected subjects (those who returned their forms), and that there were only 99 new incident cases of dementia and even fewer new cases of Alzheimer disease. Recent studies in a mouse model of Alzheimer disease support these concepts.25 In the future, it will also be important to determine whether combined dietary supplementation with DHA can decrease further mental deterioration in patients with established dementia.
METHODS
SUBJECTS
The Framingham Heart Study is a longitudinal population-based study in which subjects have been examined every 2 years.15 The dementia study began at examination cycles 14 to 15, when participants underwent a neuropsychological test battery (age range, 55-88 years).1 At the 20th biennial examination (1986-1988), 1921 subjects from this cohort were alive and free of dementia. Of these subjects, 1208 (62.9%) underwent the 20th examination and had at least 1 year of follow-up data. Plasma samples were available for the measurement of PC fatty acid content in 899 subjects (74.4% of those examined). These 899 subjects constituted our study population. The study population was 36.5% male, with a mean (SD) age of 76 (5) years, and 68.2% were high school graduates. The remaining 1022 subjects who were not part of this analysis were 36.8% male, with a mean (SD) age of 79 (7) years, and 65.9% were high school graduates. Therefore, those who were not part of this examination were older by an average of 3 years. Informed consent was obtained from all of the participants. The study was approved by the institutional review board for human research at the Boston University School of Medicine.
DIAGNOSIS OF INCIDENT DEMENTIA AND ALZHEIMER DISEASE
Members of the dementia cohort have been monitored for the development of stroke and dementia since their inclusion in the cohort.1 Participants were routinely administered a screening Mini-Mental State Examination at each biennial examination.16 Persons who scored below education-based cutoffs or who experienced a decline of 3 or more points on the Mini-Mental State Examination from the most recent previous examination were called back for a neurological and neuropsychological examination. For each case of possible dementia, a detailed case review was undertaken by a panel consisting of at least 2 neurologists (including P.A.W.) and a neuropsychologist (R.A.). The panel determined the type of dementia and the date of diagnosis using serial neurological and neuropsychological assessments, a telephone interview with a family member or a caregiver, medical records, and imaging study results. The diagnosis of dementia was made according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.17 Only persons with a duration of symptoms longer than 6 months and a score for severity of dementia of 1 or higher on the Clinical Dementia Rating Scale were considered incident cases.18 Patients with suspected cognitive deficits for whom definite dementia could not be established underwent annual reassessment. Alzheimer disease was diagnosed when subjects met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association for definite, probable, or possible Alzheimer disease.19 Subjects who had a stroke during the follow-up period were not excluded. Non-Alzheimer disease types of dementia included multi-infarct dementia, dementia complicated by stroke, nonprogressive dementia caused by brain injury, probable dementia of Parkinson disease, and other less common conditions. All confirmed cases of dementia after the 20th biennial examination (through December 31, 2002) were included, providing longitudinal follow-up of up to 16 years, with a mean of 9.1 years. All subjects with evidence of dementia before or at the 20th examination were excluded from this prospective follow-up analysis.
DIETARY MEASURES
A semiquantitative 126-item food frequency questionnaire was used to assess dietary DHA and fish intakes.20 The questionnaire was mailed to the participants before the 20th biennial examination, to be filled in and brought back at the examination. Questionnaires resulting in total energy intake of less than 600 calories or more than 4200 calories (n = 11) were excluded. Fish intake was evaluated in servings per week. Dietary data were available for 488 participants (54.3% of the study population).
BLOOD SAMPLING, APOLIPOPROTEIN E GENOTYPING, MEASUREMENT OF PLASMA HOMOCYSTEINE CONCENTRATIONS, AND FATTY ACID ANALYSIS
Blood was obtained in 0.1% EDTA at the 20th examination. Apolipoprotein E genotyping was performed using DNA isolated from blood cells and carried out as previously described.3 Total plasma homocysteine concentrations were determined with the use of high-performance liquid chromatography with fluorometric detection.4 The coefficient of variation for this assay was 9%. Plasma lipids were extracted and the phospholipids were separated as previously described.21-22 The PC fraction was subjected to fatty acid analysis by capillary gas chromatography, and fatty acid content was quantified by digital integration as previously described,23 with the coefficients of variation being below 10% within and between runs.
STATISTICAL METHODS
Cox proportional hazards regression analyses were performed to assess the relationship between plasma PC DHA and incident dementia (SAS statistical software; SAS Institute Inc, Cary, NC). The relative risk (RR) of all-cause dementia in subjects with baseline plasma PC DHA levels in the upper quartile compared with subjects with levels in the lower 3 quartiles was estimated after controlling for potential confounding factors. The proportional hazards assumption of the models was tested and was found to be satisfied. The relationship of plasma PC DHA with dementia was also studied per increment of 1 SD in the log-transformed baseline plasma PC DHA value, as well as across baseline quartiles of plasma PC DHA levels (quartiles 2 vs 1, 3 vs 1, and 4 vs 1). Similar analyses were performed for the risk of Alzheimer disease (subjects in whom other types of dementia developed during follow-up were censored at the date of the diagnosis of dementia). In addition, risks of dementia and Alzheimer disease were studied according to baseline dietary DHA and fish intakes, following the same statistical analysis as that described for plasma PC DHA. Identical analyses for other fatty acids were also performed.
|
|
| |
| |
|
|
|