 |
 |
 |
| |
Efavirenz Nonadherence in Blacks & Whites
|
| |
| |
Note from Jules Levin; Below are two reports on the study presented in Toronto at IAC on the effects of nonadherence to efavirenz for whites and blacks in ACTG study 5095. This substudy found that nonadherence by blacks than whites was more likely to result in viral failure for blacks than whites. Immediately below is the report by Mark Mascolini for NATAP on this poster, and this is followed below by the actual poster and data in it reported by Bruce Schackman and the ACTG 5095 study team. Several possible explanations for these findings are considered in the two reports below, but we may need further consideration and examination of what happened in this study to understand the results. Due to the long half-life of efavirenz as well as other drugs with a long half-life these drugs stay in the blood for a prolonged period of days to 1-2 weeks after stopping the drug. Previous research has reported that after stopping efavirenz it remains in the blood for a longer time for people with certain genes and some blacks may be more likely to have these genes. Further studies are required to evaluate this question.
Efavirenz nonadherence may pose greater risk for blacks
IAC Toronto Aug 2006
Mark Mascolini
Despite the convincing evidence of efavirenz potency regardless of HIV disease stage (see preceding section), one factor, race, may weigh against a good response to this NNRTI--at least in people who adhere poorly to their regimen. That possibility rests on yet another analysis of ACTG A5095, this one presented by Bruce Schackman (Cornell University, New York) [8]. The finding that shaky adherence inflates the risk of virologic failure more in blacks than whites taking efavirenz could have global portent if it applies to any blacks taking NNRTIs, not just to the African Americans studied in ACTG A5095.
ACTG researchers had already reported that efavirenz regimens faltered in blacks more than whites and that blacks enrolled in this study had worse adherence than whites. The new analysis untangled the links between race, efavirenz failure, and adherence by measuring adherence in four ways:
- 4-Day recall: reporting not missing any doses of any active agent during the 4 days before the evaluation
- Weekend: reporting not missing any doses of any active agent during the weekend before the evaluation
- Last month: reporting not missing any doses of any active agent within the last month
- Never missed: reporting not missing any doses of any active agent during the 4 days before the evaluation AND reporting never skipping medications
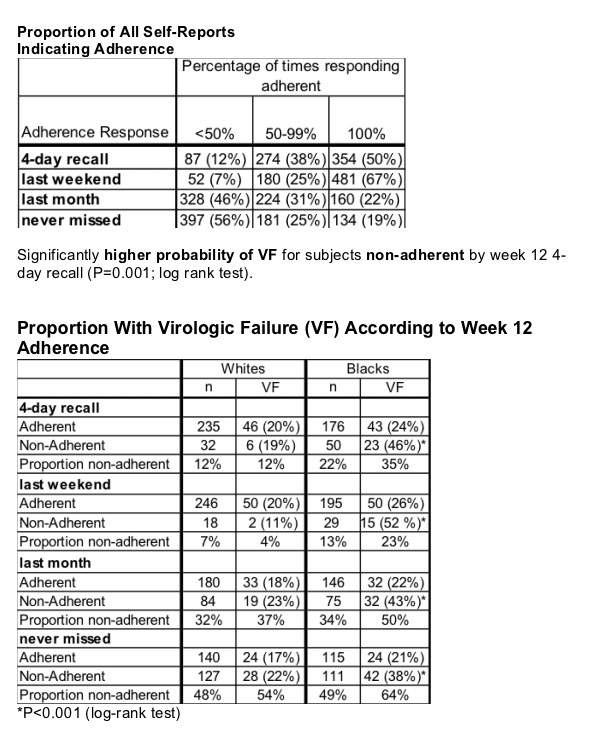
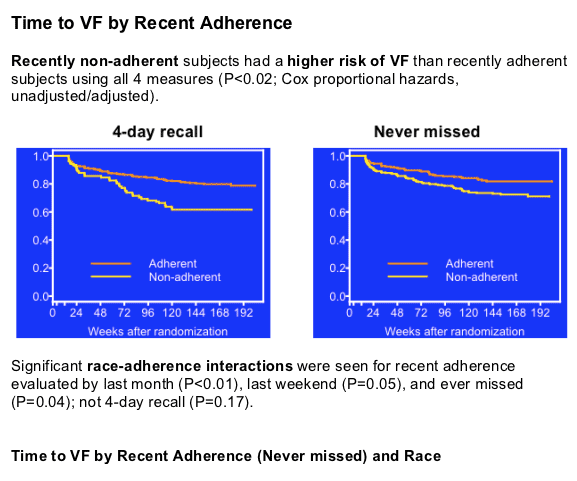
Overall adherence proved better with the less stringent pill-taking meters, 4-day recall (84%) and last weekend (90%) and worse with the more stringent measures, last month (67%) and never missed (48%). Defining failure as a confirmed load above 200 copies, Schackman charted a higher failure rate in people with recent nonadherence than in those with recent good adherence by all four adherence tests (P < 0.02).
No surprise there, but the analysis also showed that efavirenz flopped in significantly higher proportions of nonadherent blacks than nonadherent whites. Among 32 whites nonadherent by 4-day recall, only 6 (19%) ended up in virologic failure. But among 50 blacks who reported poor adherence by 4-day recall, 23 (46%) had a virologic failure, a highly significant difference from nonadherent whites (P < 0.001). Eighteen whites scored themselves nonadherent on the weekend test, and efavirenz failed in 2 of them (11%), compared with 15 of 29 blacks (52%) reporting nonadherence on that test (P < 0.001).
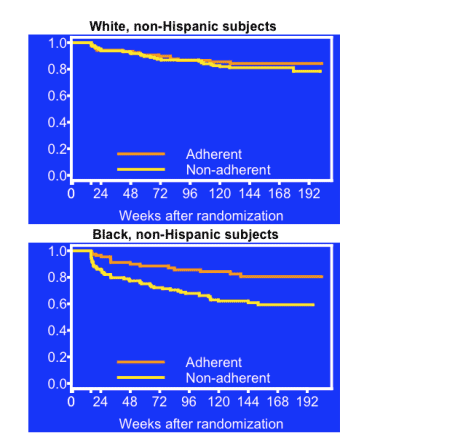
Efavirenz failure comparisons by the other two adherence barometers also significantly favored whites. On top of that, time to virologic failure proved significantly faster when comparing nonadherent blacks with adherent blacks, but not when comparing nonadherent whites with adherent whites, according to the last-month test (P < 0.01), the never-missed test (P = 0.04), and the last-week test (P = 0.05).
Why poor adherence threatens nonadherent blacks so much more than nonadherent (or adherent) whites remains a mystery, but the ACTG team has some hypotheses. Answering an e-mail query, Cornell University's Roy Gulick, A5095's principal investigator, listed three possibilities that may merit future study: (1) differences in adherence patterns between blacks and others, for example, missing an occasional dose versus missing all doses for 1 week, (2) differences in low-level side effects causing differential toxicity and resulting in partial adherence, and (3) differences in social supports.
The worse virologic response in blacks versus whites with poor adherence is doubly surprising because other work suggests that a genetic difference between blacks and whites results in higher efavirenz levels among blacks [9] (though that may cause the possible race-based side effect difference that Gulick mentioned). Other recent research indicates that people can get away with wobbly adherence to NNRTIs more easily than subpar adherence to the unboosted PIs indinavir or nelfinavir [10,11]. A just-published study of 110 marginally housed people that measured adherence by unannounced pill counts found that most people taking efavirenz or nevirapine controlled viral replication with at least a 54% adherence rate, while it took 73% adherence for most people taking a PI to stifle HIV [11].
Analyzing these studies, A5095's chief, Roy Gulick, proposed that "sustained virologic suppression even with some degree of imperfect adherence" to NNRTIs probably reflects several factors, "including the inherent antiretroviral potency of NNRTI drugs (in combination with nucleoside analogues), as well as their convenience, tolerability, and long plasma drug half-lives" [12]. Gulick suggested this greater "forgiveness" with efavirenz or nevirapine probably also applies to ritonavir-boosted PIs.
Do blacks have more side effects with efavirenz?
The earlier ACTG study confirming higher efavirenz levels in blacks than whites also found more central nervous system (CNS) side effects 1 week after starting efavirenz in people with a genotype more common among blacks [9]. At the Toronto meeting analysis of a different trial disclosed no greater risk of CNS toxicity in blacks than in whites or Hispanics, though Hispanics suffered dizziness more than the other groups [13]. Virologic response to efavirenz did not differ significantly by race or ethnicity in this trial, which compared a switch to efavirenz with a continued twice-daily nonefavirenz regimen. A 48-week noncompleter-equals-failure analysis charted a statistically equivalent 77% sub-50-copy response rate among 66 blacks, an 82% response among 73 whites, and an 82% response among 60 Hispanics.
Looking at all grades of central nervous system side effects [see note 14], this US team found that 14 blacks (21%), 13 whites (18%), and 17 Hispanics (28%) had one or more such problems, though differences between groups were not statistically significant. Hispanics experienced dizziness significantly more often (20%) than blacks (6%) or whites (9%) (P < 0.05 versus whites). Considering only grade 2 to 4 side effects, these researchers again found statistically similar overall rates in blacks (8%), whites (8%), and Hispanics (13%), but significantly more dizziness in Hispanics (10%) than in blacks (0%) or whites (2%) (P < 0.05 versus whites).
Racial Differences in Self-Reported Adherence and Association With Virologic Failure on Efavirenz-Containing Regimens for Initial HIV Therapy: Results from ACTG 5095
Reported by Jules Levin
IAC Toronto Aug 2006
Poster # TUPE0113
B.R. Schackman1, H. Ribaudo2, A. Krambrink2, V. Hughes1, D.R. Kuritzkes3, R.M Gulick1 for the ACTG 5095 Team
1Weill Medical College of Cornell University, New York, NY, USA; 2Harvard School of Public Health, Boston, MA, USA; 3Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA
A5095 STUDY DESIGN
Double-blind, randomized, placebo-controlled study at U.S. ACTG sites:
-- ZDV/3TC + EFV (2NRTI + EFV)
-- ZDV/3TC/ABC + EFV (3NRTI + EFV)
-- ZDV/3TC/ABC (3NRTI)
Study population
- HIV-1-infected subjects with no prior antiretroviral treatment
- HIV-1 RNA >400 c/ml; any CD4 cell count
- Stratified by screening HIV-1 RNA: < or >100,000 c/ml
All subjects to be followed regardless of treatment change for 96 weeks after last randomization.
A5095 HISTORY
-- 1147 subjects randomized between Mar. 2001 and Nov. 2002
-- ZDV/3TC/ABC stopped by the DSMB in Feb. 2003
Inferior to each of the 2 EFV-containing regimens in terms of rates and time to virologic failure (VF) (Gulick, et al, NEJM 2004;350:1850-61)
-- Following an amendment to extend follow-up, EFV-containing arms completed follow-up in Mar. 2005
-- No difference between 2 or 3 NRTI + EFV over 3 years median follow-up
-- Black/non-Hispanic patients had
increased risk for VF and higher proportions of non-adherence at weeks 4 and 12
(Gulick, et al, JAMA 2006;296:769-81)
METHODS
Adherence evaluated by self-report at weeks 4, 12, 24 and every 24 weeks
thereafter.
Based on self-report, adherence defined for four metrics as follows:
-- 4-day recall: reporting not missing any doses of any active agent during the 4-day period prior to evaluation
-- weekend: reporting not missing any doses of any active agent during the weekend prior to evaluation
-- last month: reporting not missing any doses of any active agent within the last month
-- never missed: reporting not missing any doses of any active agent during the 4 days prior to evaluation AND reporting "Never Skip" medications
For each adherence measure:
-- Proportions of subjects categorized as adherent over time were calculated
-- Association of adherence and VF (confirmed VL>200 c/ml) evaluated using Kaplan-Meier, log-rank tests, and Cox proportional hazards models
(adjusted/unadjusted for baseline and treatment covariates):
- Fixed time covariates used for adherence evaluated at week 12
- Time-dependent covariates examined the effect of "recent" adherence (defined as the adherence evaluation closest to a given event time and no more than 24 weeks prior to that event)
RESULTS
The average proportion of adherent responses was 84% for 4-day recall; 90% for last weekend; 67% for last month; and 48% for never missed.
CONCLUSIONS
Non-adherence is consistently associated with virologic failure on efavirenz-containing regimens using a 100% self-reported 4-day adherence measure as well as stricter (last month, never missed) and less strict (last weekend) measures. There is evidence of an adherence-race interaction over time on efavirenz-containing regimens, with a greater effect of non-adherence on virologic failure with blacks than whites.
PHARMACEUTICAL CO-SPONSORS: Boehringher-Ingelheim; Bristol-Myers Squibb;GlaxoSmithKline
|
| |
|
 |
 |
|
|