 |
 |
 |
| |
First-Line NNRTIs Beat PIs in a 5-Year Trial, But . . .
|
| |
| |
Mark Mascolini
XVI International AIDS Conference, Toronto
August 15, 2006
People starting therapy with a nonnucleoside (NNRTI) regimen rather than a protease inhibitor (PI) combination had a better virologic response after 5 years of follow-up [1]. But because the trial recruited its 1397 participants from 1999 through 2002-and because clinicians could pick which drugs to use after randomization-74% of people in the PI group did not use a ritonavir boost, which is the current PI standard of care.
When an NNRTI regimen did fail virologically, that failure left people with mutations to two antiretroviral classes more often than PI failure did. And the FIRST trial found no difference between a first-line PI and an NNRTI in a composite endpoint linking CD4 decline, progression to AIDS, and death (the study's primary endpoint).
Conducted at sites across the United States, FIRST also confirmed that a triple-class regimen is not more potent than a standard two-class combo. In fact, significantly more people starting a three-class combination had to stop the regimen because of side effects.
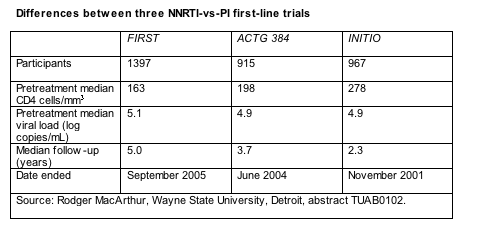
In certain ways FIRST does little more than echo results of two other randomized comparisons of first-line NNRTIs and PIs, ACTG 384 [2,3] and INITIO [4]. FIRST is bigger than those two studies and had longer follow-up when first reported (Table). But the main difference between the studies is that people in ACTG 384 and INITIO had to take efavirenz or nelfinavir or both with their nucleosides. In FIRST clinicians and participants picked their own drugs after being assigned to one of the treatment arms. While 61% in the PI group chose nelfinavir and only 26% picked a boosted PI; 63% in the NNRTI arm opted for efavirenz and 36% for nevirapine. FIRST also recruited people with more advanced disease than the other two trials (Table).
FIRST enrollees had a relatively young median age of 38 years (interquartile range 32 to 44), 54% were black, 17% Hispanic, and 21% women or girls. (People had to be 13 or older to sign up.) Slightly more than a third already had an AIDS diagnosis, and 20% had hepatitis C virus coinfection. Only 9.7% of study participants stopped coming back for follow-up visits, a swell record for such a long trial.
After a median 5 years of follow-up, curves describing the composite endpoint for each treatment arm did not vary from each other by a hair's breadth. For unclear reasons, Hispanics had a higher AIDS or death rate with PIs (8.5 per 100 person-years) than with NNRTIs (5.6), while whites had a higher rate with NNRTIs (5.6) than with PIs (4.2). Progression rates did not differ with treatment assignment for blacks.
Risks of AIDS, death, a CD4 count under 200 cells/mm3, a grade 4 toxicity, or discontinuation of an antiretroviral because of toxicity were all higher in the triple-class group than in the double-class groups. Whereas 43.2% randomized to the PI arm and 31.7% randomized to an NNRTI switched treatment strategies at least once, 79.5% randomized to three-class therapy changed strategies. Median time to a first switch in treatment strategy was longer in the NNRTI group (23.5 months) than in the PI group (18.2 months) or the triple-class group (8.8 months).
The percentage of people who kept their viral load under 50 copies/mL proved significantly lower in the PI arm than in the NNRTI group or the triple-class group. Compared with people who started a PI regimen, those starting an NNRTI had a 1.63 times higher chance of staying below 50 copies/mL (95% confidence interval 1.36 to 1.95). Again, that result must be interpreted in light of predominant nelfinavir use in the PI arm.
Genotypes of people with virologic failure showed similar single-class resistance rates with a PI (31.3%), an NNRTI (31.4%), or a three-class regimen (38.4%). But failure of an NNRTI resulted in more multiclass resistance (22.3%) than did failure of a PI (13.8%) or a three-class combo (14.2%).
ACTG 384 sounded the death knell for first-line nelfinavir at the 2002 International AIDS Conference in Barcelona. INITIO confirmed the first-line superiority of efavirenz over nelfinavir. So it was unlikely that FIRST, whose participants relied mainly on efavirenz in the NNRTI arm and on nelfinavir in the PI arm, would diverge from that finding. One could certainly argue with the FIRST team's conclusion that an NNRTI, a boosted PI, or an unboosted PI offers a similar shot at good initial management of HIV infection.
Another randomized trial to be unveiled at this conference later this week compared two current standard first-line drugs, efavirenz and lopinavir/ritonavir [5]. Also, a US cohort study presented at the meeting found more durable virologic suppression with efavirenz than with a boosted PI [6]. I review the cohort study in a separate piece dated August 15 on the NATAP site (see "More Durable HIV Suppression With Efavirenz Than Boosted PIs"), and I will review the randomized trial on Thursday.
References
1. MacArthur RD, Novak RM, Peng G, et al. Long-term clinical and immunologic outcomes are similar in HIV-infected persons randomized to NNRTI vs PI vs NNRTI + PI-based antiretroviral regimens as initial therapy: results of the CPCRA 058 FIRST study. XVI International AIDS Conference. August 13-18. Toronto. Abstract TUAB0102.
2. Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med 2003;349:2293-2303.
3. Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med 2003;349:2304-2315.
4. Yeni P, Cooper DA, Aboulker JP, et al. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet 2006;368:287-298.
5. Riddler SA, Haubrich R, DiRienzo G, et al. A prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV infection--ACTG 542. XVI International AIDS Conference. August 13-18. Toronto. Abstract THLB0204.
6. Sterling T, Barkanic G, Raffanti S, et al. Does optimal timing of HAART initiation differ with newer treatment regimens that include efavirenz or ritonavir-boosted protease inhibitors? XVI International AIDS Conference. August 13-18. Toronto. Abstract TUPE0205.
|
| |
|
 |
 |
|
|